Quick Links
IMPORTANT UPDATES:
POLICY IRB-01 UPDATED:
Policy IRB-01 has been updated and posted on the Policies and Guidance website. A summary or revisions and a version comparison report are available on this site. The direct links are provided below:
- SUNY Downstate Health Sciences University, Human Research Protections Program, Policy and Procedures (IRB-01)
- Summary of Revisions
- Version Comparison Report
STEP 11 UPDATED:
Form 11-4C has been updated and finalized. This revised form includes digital signatures to help Project Leads more efficiently collect required signatures before submitting the form in IRBNet. This form must be completed for ALL non-human research and all non-research projects carried out by residents and may also be used by students or other members of the Downstate workforce if required by their Department or College/School.
See Step 11 and 11A on the IRB submission website for full details.
For tips on how to fill and digitally sign a fillable PDF, see IRB Submission Tip #2 (below).
COI TRAINING AND REPORTING REQUIREMENTS:
A NEW IRB Procedure has been established for COI Disclosures and Training Requirements. This is posted in the section on IRB Guidance for Investigators on the IRB Policy and Guidance website.
KINGS COUNTY RESEARCH:
STEP 18 has been updated to include clarifications and new requirements for Kings County Research.
ARTIFICIAL INTELLIGENCE GUIDANCE:
Two new resources are now posted on the IRB Policy and Guidance website:
- To support Downstate investigators conducting research that involves artificial intelligence (AI), the Downstate IRB recommends reviewing the Framework for Review of Clinical Research Involving AI, developed by the MRCT Center and WCG (click to Download). This resource provides practical guidance on addressing key ethical and regulatory challenges, such as algorithmic bias, adaptive learning, security, privacy, and human oversight, all which are important for designing studies that include AI. The framework includes tools like a regulatory decision tree, stage-specific guidance, and ethical considerations to help ensure thorough, compliant, and responsible research. The Downstate IRB will use this framework when conducting reviews of human research submissions that use AI. Access to the full framework and additional resources are available at: MRCT Center AI & Ethical Research Framework.
- Statewide ban of DeepSeek AI application.
STEP 7B UPDATED:
- Medical device trials that require an IDE.
- Drug or biologic trials that require an IND.
- Clinical trials deemed more than minimal risk.
- Clinical research involving bedside infusions, pregnant women, fetuses, children, or cognitively impaired adults.
- Clinical research that prospectively enrolls UHD or DHP patients, or utilizes UHD or DHP resources, unless the sole interactions or interventions are surveys or obtaining consent.
Clinical research of the above types, funded and supported by UHD or DHP, necessitates the respective approval of each organization.
See Step 7 on the IRB submission website for full details.
IRB Submission Tips:
| Go to the step below: | To find one of the following forms, templates, policies, or guidance: |
|---|---|
| Tips: |
Review the tip sections to understand: Tip 1: Steps and associated materials Tip 2: Completing fillable PDF forms and obtaining digital signatures Tip 3: Delegation of Signatures Tip 4: Ensuring effective and efficient IRB approval process. Tip 5: Successful submission of Form 11-A4 All IRB forms and templates are available on a separate website for each reference. Click here for the Forms & Templates website. |
| Step 1: |
Policies and guidance |
| Step 2: |
Tips for planning the project |
| Step 3: |
PI Status |
| Step 4: |
Downstate workforce |
| Step 5: |
Determining which IRB to use Agreements IRB Fees |
| Step 6: |
Training Conflict of Interest disclosures |
| Step 7: |
Protocol templates CMRC Review Request Form Ancillary review process and forms for certain types of unfunded clinical research |
| Step 8: |
Obtaining legally effective informed consent and HIPAA research authorization Consent Templates Stand-alone HIPAA Authorization Pregnancy follow-up consent SUNY RF Payment Consent, Waiver, and Guidance Assent Recruitment authorization forms Medical release form HIPAA waiver/alteration form Waiver of informed consent requirements form |
|
Step 9: |
Obtaining legally effective informed consent and HIPAA research authorization Short forms Translation certificates |
| Step 10: |
Additional IRB materials to submit with IRB application |
| Step 11: |
IRB Application forms for initial review |
| Step 12: |
IRBNet guidance |
| Step 13: |
Scientific review guidance and forms |
| Step 14: |
Ancillary reviews |
| Step 15: |
Department Chair/Dean approvals |
| Step 16: |
IRBNet |
| Step 17: |
Responding within IRB deadlines |
| Step 18: |
Completing requirements for external sites |
| Step 19: |
Post-IRB approval requirements |
| Step 20: |
Post-IRB application forms |
| Step 21: |
Quality Assessment Program forms |
Note: Instructions may vary due to the software version or computer settings. Please consult the Downstate Help Desk for questions about computer settings or software.
Step-by-Step Instructions:
1. Saving the Downstate IRB Form on the desktop:
-
- Download the applicable Downstate IRB Form from the applicable Step from the IRB Submission web page.
- Before filling out the form, save the form on your computer with a descriptive file name, such as "8383730_survey4_exempt-application_01.15.2023"
- You may now close the form so that it may be imported into Adobe Reader as indicated below.
2. Complete the form:
-
- Open Adobe Reader on your computer. Note: The "Adobe Reader" software is available for free download at Adobe Reader Download website or you can search for it within the Adobe website.
- The form must be filled out properly in Adobe Reader to permit the use of dynamic functions such as scrolling and the e-signature feature.
- Navigate to "Tools" and click on the "Fill & Sign" icon.
- Click on "Select a File", navigate to the form, select it, and click open. The form can now be viewed and completed.
- Fill out the form by clicking on the fields and entering the required information.
- Review and edit any responses to ensure accuracy.
- A digital signature is required on certain Downstate IRB forms (e.g., HIPAA Waiver, Individual Investigator Agreement, Honest Broker (to be updated), Delegation of Signature (to be updated). Please review step 3 below to obtain a digital signature.
- Save the completed form on your computer and rename the file if necessary.
- Forward the form for signature, when applicable.
3. Digital Signature:
The IRB incorporates “Digital Signatures” in the forms which require electronic signatures. A digital signature is an e-signature that is generated using a digital certificate and cryptographically bound to the document using public key infrastructure (PKI). Digital signatures comply with regulatory requirements around the world and provide the highest level of identity assurance with digital documents. Please note that this is NOT a signature fillable block that is sometimes seen on PDF forms where you could just type or draw your signature.
-
- If you received the document from someone else, complete the above steps first.
- Be sure you navigate to "Tools" and click on the "Fill & Sign" icon; otherwise, you will not be able to see the signature box on the form nor be able to execute a digital signature.
- Review form to ensure content is correct before signing it.
- Make any necessary changes and save it on your computer.
- Navigate to the signature section of the document.
- DO NOT CLICK THE “Sign yourself” button at the top of the adobe form, as this is NOT a digital signature.
- Click on the "Red Tab" (see image below) in the upper left corner of the signature box. Adobe will prompt you to sign the document. Follow the instructions to establish a digital certificate if you do not have one set up on your computer.
4. Submit the completed form to the IRB using IRBNet.
The following tips are offered to help ensure an effective and efficient IRB approval process:
- Complete all required application materials, forms, and templates.
- Review IRB policy, guidance, templates as needed.
- Always contact the IRB Office if you are unsure about something. We are here to help you.
- Make sure all materials submitted to the IRB are consistent and congruent with one another.
- Obtain all required signatures.
- Ensure all required training and conflict of interest disclosures are complete.
IRB Submission Steps:
Below is a step by step process for preparing and submitting an IRB application along with all related materials to seek Downstate IRB approval or activation of human research activities.
The order of these steps is designed for a new investigator and are not meant to be prescriptive and many of the steps can take place in parallel to save time. A more experienced investigator or coordinator who has a good grasp on the IRB process may wish to carry out the steps in a different order.
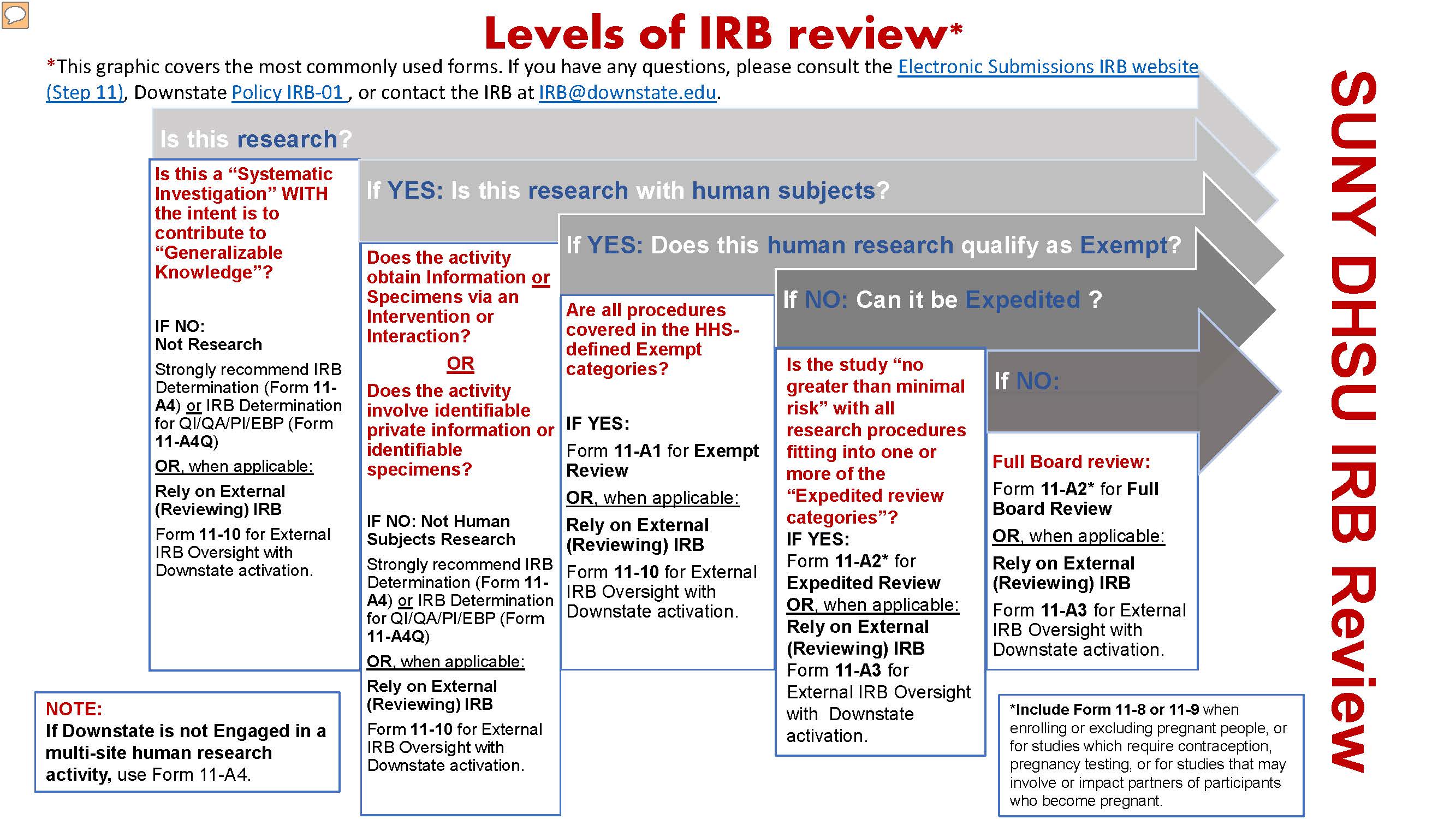
Note: For more information on whether an activity requires IRB approval, please refer to the Downstate IRB FAQs. To request an IRB determination letter for activities which do not require IRB approval, skip to step 11 (below) and review the information on the IRB Decision Aid forms.
Review the Downstate IRB website for instructions and details on how to submit an IRB application.
Refer to the Policy and Guidance to understand Downstate Policy IRB-01 and other applicable policies and IRB guidance.
TIPS:
- Start early!
- Establish the study team and meet often to keep the project flowing.
- Investigators performing certain clinical interventions must have appropriate privileges, as determined by the Department Chair in accordance to the Downstate Medical and Dental By Laws of the Medical and Dental Staff.
- Have a kick-off meeting.
- Establish goals and critical milestones.
- Assign tasks.
- Conduct a literature search and keep a bibliography of references to include with the protocol.
- Consult with a mentor and other experts in the field, as needed.
- Consult with a biostatistician, as needed.
- When seeking funding, work with the Sponsored Programs Administration to establish the award. This can be done in parallel; however, the research cannot begin until both the IRB has approved the study and it is funded.
- Determine if the research must be registered on www.clinicaltrials.gov and develop a plan for complying with reporting requirements.
- Make a plan for any required ancillary reviews, CMRC reviews, Department/Dean & PI signatures.
- Make a budget and plan for IRB fees.
Effective on February 20, 2020, the IRB requires the PI to submit a current CV, resume, or NIH Bio-sketch with each new study submitted to the IRB.
Effective on February 3, 2021: A new or inexperienced PI must include a mentor on the IRB application.
The PI must have "PI Status" which is defined as:
- Downstate Faculty (including those with Emeritus Status and those under recruitment)
- Kings County Clinician
- Individual who qualifies to be a PI at an external site, when Downstate is engaged in human research (e.g., Federal funding or support is provided to Downstate, or Co-investigators or key personnel on the study are members of the Downstate workforce)
Notes:
- In certain situations, multiple Co-PIs are permitted. Consult Policy IRB-01 to determine if Co-PIs can be used for the particular project.
- The Site Principal Investigator for a study conducted at NYC H+H, Kings County must be a full-time, part-time or voluntary physician who is a member of the Medical Staff at Kings County and who has appropriate clinical privileges as defined in the Facility's Medical Staff Bylaws. This individual must also be approved by the reviewing IRB as personnel on the study.
-
All studies entered into STAR at Kings County Hospital require a site Principal Investigator (PI) that is a NYC H+H/Kings County Hospital attending physician or staff member with > 60% salary commitment to NYC H+H/Kings County Hospital (majority salary). This designated site PI must be included as an investigator approved by the SUNY Downstate IRB to work on the study.
- A PI with PI Status is required for Forms 11-A1, 11-A2, 11-A3, 11-A5, 11-8, 11-9, and A1B.
PROJECT LEADS:
A “Project Lead” for Form 11-A4 or Form 11-10 is not required to have "PI Status"
and may e-sign the IRBNet submission as "Other Signatory" or “Principal Investigator”.
A Fellow, Resident, or Student may serve as a “Project Lead.”
Notes:
1) Certain students are not permitted to conduct human research projects based on
their program requirements. For example, a DNP Student in the College of Nursing may
not conduct human research as part of their program. If it is determined their project
meets the definition of human research, including exempt research, the project must
be redesigned to meet program requirements.
2) If a project that was submitted for a non-human research determination is determined by the IRB to represent a human research activity, Form 11-A4 cannot be used. All human research activities musts be reviewed and approved by the IRB using the applicable form.
Understanding whether a PI, Co-PI, Co-Investigator, or Key Personnel is a member of the “Downstate workforce “or an "External investigator" will help determine:
- which IRB(s) will have oversight of the research,
- which training and Conflict of Interest (COI) disclosures are required,
- whether an IRB Reliance Agreement (IRA) or an Individual Investigator Agreement (IIA) must be established, and
- whether any additional agreements are required.
Understanding whether a PI, Co-PI, Co-Investigator, or Key Personnel is a member of the “Downstate workforce “or an "External investigator" will help determine:
- which IRB(s) will have oversight of the research,
- which training and Conflict of Interest (COI) disclosures are required,
- whether an IRB Reliance Agreement (IRA) or an Individual Investigator Agreement (IIA) must be established, and
- whether any additional agreements are required.
For the purposes of the Downstate IRB, the Downstate workforce include investigators who act on behalf of Downstate, including, but not limited to:
- Faculty members, employees, and staff who are paid by Downstate or the Research Foundation (RF) for SUNY, working on behalf of Downstate,
- Collaborators who are paid with a subaward with the Research Foundation for SUNY, working on behalf of Downstate,
- Individuals with a Downstate Voluntary Faculty appointment with medical privileges (credentialed by University Hospital at Downstate),
- Retired Downstate faculty member with emeritus status,
- Residents, Fellows, or Medical Students who are sponsored by Downstate
- A sponsored Resident or Fellow is any Resident or Fellow who is part of a Downstate residency/fellowship training program who goes through the Downstate GME Office, regardless of rotation site or employment status.
- A sponsored Medical Student who is sponsored through one of the Downstate colleges or through an affiliation agreement.
- Students in a Downstate academic program,
- Downstate Research Volunteers (onboarded by the Senior VP of Research Office).
- All students and trainees who are NOT in a Downstate academic program MUSTgo through the Senior VP of Research Office to be onboarded as a Research Volunteer to become members of the Downstate Workforce. Note: Former external students covered under an existing executed Individual Investigator Agreement may continue to conduct research on existing studies.
- Research Volunteers are agents of the Downstate Workforce, but are not considered to be employees; therefore, Downstate cannot review their COI disclosures or develop managing plans for Significant Financial Interests.
CAUTION: If a sponsored Resident, Fellow, Medical Student, or Student is an employee or agent of another institution, (s)he should check to see if their IRB or Institution has any additional requirements for their review and/or whether they require an IRB Reliance Agreement (IRA) or Individual Investigator Agreement (IIA), particularly if they are conducting the research on behalf of their other institution. However, even when an IRA or IIA is established for an investigator who is sponsored by Downstate, (s)he is still responsible for following Downstate policy and completing Downstate requirements for training and COI (if applicable).
-
The following individuals are considered "External Investigators" and are not members of the Downstate workforce:
- External consultants (e.g., those paid by sponsors or other entities outside of Downstate),
- Independent Contractors, as determined by RF HR based on IRS Regulations and the RF Engaging Independent Contractors procedure.
- Individuals with Voluntary Faculty appointments at Downstate without medical privileges, and
- External residents, fellows, and students (e.g., those without an affiliation agreement with Downstate).
- Employees or agents of institutions that are not listed as components of the Downstate Federal Wide Assurance (FWA) on file with HHS OHRP, including
- University Physicians of Brooklyn (UPB),
- NYC Health + Hospital, Kings County Hospital,
- Companies within the Downstate Biotech Park,
- Other institutions, or
- Private practices.
NOTES:
1) An external investigator must establish an Individual Investigator Agreement (IIA) with Downstate or be covered under an IRB Reliance Agreement (IRA) between their institution and Downstate for the Downstate IRB to approve their research activities. See Step 5F below, for additional information.
2) Independent Contractors may also be required to establish a BAA, DUA, or other types of agreements when applicable to the research.
3) For the of the purposes of the Downstate IRB, an investigator cannot have an independent contractor relationship with the RF and simultaneously be considered a member of the Downstate Workforce.
External Investigators (those who are not part of the Downstate workforce) must follow their Institution’s requirements.
- For Exempt Review or IRB Determinations (e.g., not human research, not engaged, etc.), External Investigators must follow their Institution’s requirements and should not be included on the Downstate IRB application. If their Institution does not have an IRB to make these determinations, an IRA or an IIA may be established with the Downstate IRB to make these determinations.
- For Expedited or Full Board reviews, when External Investigators conduct human research activities that make their institution engaged in human research per OHRP guidance, they may use their Institution’s IRB or the Downstate IRB (if an IRA or IIA is established).
- A Single IRB (sIRB) is required for oversight of any federally funded or conducted study. The PI must obtain approval in advance to use the sIRB and include sIRB fees in the award budget for the review of all sites.
- CAUTION: The Downstate IRB cannot be used as a sIRB for a federal funded or conducted study. See Step 5 below, for additional information.
- When an Application for External IRB oversight is submitted to the Downstate IRB, only the Downstate workforce can be included. External investigators (including KC and UPB) must consult their institution and the external IRB for guidance to be included in their approval process, as the Downstate IRB does not have jurisdiction over External Investigators approved by an External IRB.
Note: For applications for either Expanded Access for Treatment or a HUD for Clinical Purposes, Clinicians from Kings County or UPB may be included on the Downstate IRB application. Other External Clinicians should seek approval from their Institution’s IRB.
See “Step 5F” (below) for additional details for required agreements.
NOTES:
- CAUTION: Do not confuse the term “Investigator” for “Investigator for the Purposes of COI”(see step 6 below).
- The Downstate Financial Conflict of Interest Committee cannot review COI determinations of those who are not employed by Downstate; therefore, an Investigator who is also an “Investigator for the Purposes of COI” and not an employee of Downstate must provide a COI adjudication letter and documentation that they have completed their required COI training from their employer with a statement that the employer follows NIH COI policies. If their employer does not have jurisdiction or follow NIH COI policies, the investigator should indicate this in the IRB application seek these requirements from an external COI Committee, Attorney, or Consultant who confirms they follow NIH policies.
- Individuals (including consultants) who perform non-human research activities or those who do not conduct activities that do not make their institution engaged in human research do not require Downstate IRB approval (seePolicy IRB-01 for details) and should not be listed on the Downstate IRB application nor the IRBNet Registration Form as investigators; however, these individuals are encouraged to take CITI and HIPAA training and should be noted in the protocol, a protocol appendix, notation in the IRB application, or cover letter to the IRB with a description of their role on the project.
- External investigators should consult with their institution’s IRB or Human Research Protection Program or equivalent office to determine if they must comply with other requirements.
- External investigators must be covered by an Individual Investigator Agreement or an IRB Reliance Agreement (see Step 5) and may NOT become Downstate Research Volunteers for the purposes of having the Downstate Financial COI Committee review their disclosures. If they are conducting human research on the Downstate site or if they will have access to Downstate Protected Health Information or Downstate private identifiable information, they must have an IRA or IIA or another IRB may review the research.
Quick Links (referenced in the sub-steps below):
- Determining which IRB to Use, which Agreements are required, and which IRB fees to budget
- WCG IRB Connexus (new system)
- WCG IRB Connexus (past system)
- National Cancer Center Central IRB
- Biomedical Research Alliance of New York (BRANY) IRB
- SMART IRB Online Reliance System
- WCG IRB Forms
- WCG Single IRB Quote Request Form
- Advarra CIRBI Portal
-
Advarra CIRB Quick Steps to submit an Initial Protocol Application.
- Fee Schedule
Click on the link to review the flow chart for: Determining which IRB to Use, which Agreements are required, and which IRB fees to budget.
From the flowchart, determine the following:
- Determine, which IRB(s) will have oversight. This decision is generally based on the following:
- Funding source
- Whether Single IRB (sIRB) review is required by NIH, Federal Department/Agency, Common Rule, or FDA (once the FDA implements new regulations).
- Whether the sponsor or main PI of a multi-site study requires the use of a specific Central IRB of sIRB.
- The policies that the Main (overall) PI and/or other external collaborating investigator(s) must follow at the at the site where they are employed or act as an agent, when conducting multi-site human research.
- Whether investigators who are members of the Downstate workforce are conducting activities that are considered Not Research, Not Human Research or whether these activities make Downstate engaged in human research.
- Determine which agreements are required for investigators who are not members of the Downstate
workforce:
- IRB Reliance Agreement(s) (IRA)
- Individual Investigator Agreement(s) (IIA)
Note: See Step 5F below for more information about other agreements that may be required, such as: Data Agreement (DA), Data Use Agreement (DUA) for sharing Limited Data Sets (LDS), Clinical Trial Agreement (CTA), Material Transfer Agreement (MTA), Facilities Use Agreement (FUA), Confidentiality Agreement (CA), etc.
- Determine which IRB fees are required:
- Consult the Downstate IRB Guidance: Fee Schedule.
- Contact Sponsored Programs Administration (SPA) for information on the budget process and include all IRB fees in your budget.
- Research budgets must include all IRB fees:
- The PI is responsible for including all Downstate IRB fees in research budget based on whether fees are paid out of the research budget or if they will be invoiced directly to the sponsor.
- Industry sponsored research budgets are negotiated and approved by WCG Clinical on behalf of the RF.
- Invoices for IRB fees:
- WCG Clinical will automatically invoice for the local Downstate IRB fee for Industry sponsored research on behalf of the RF upon study start up.
- The Downstate IRB will notify the RF to bill any other required Downstate IRB fee.
- External Reviewing IRBs should invoice their IRB fees directly to the Industry Sponsor; however, the PI should confirm with the Sponsor and External Reviewing IRB, for each study.
- External IRBs should invoice the PI for IRB fees related to sIRB review.
- For questions about invoices for IRB fees, please contact Sponsored Research Programs.
- Research budgets must include all IRB fees:
- Downstate IRB fees:
- When an External IRB is the Reviewing IRB for an Industry sponsored study, the RF will invoice the sponsor a one time fee of $1,000 (as per budget).
- When the Downstate IRB is the Reviewing IRB for sponsored research not otherwise overseen by another external IRB, the RF will invoice the sponsor $4,000 (as per budget). However, please note that the RF does not charge any Downstate IRB fees for Downstate or Kings County intramurally funded studies or for extramurally funded studies sponsored by the government, non-profit entities, public entities, professional associations, oncology groups, or SUNY.
- In general, when an External IRB is the Reviewing IRB, the Reviewing IRB will bill an Industry Sponsor directly for their IRB fees. Confirm this information with the Reviewing IRB and the Industry Sponsor.
- A quote must be obtained from the WCG IRB for the following studies:
- Industry sponsored clinical investigations, unless already covered as noted above.
- Federally funded non-exempt human research which requires an sIRB, when Downstate is the Primary Awardee. Note: For sIRB studies, the WCG IRB will invoice the PI to be charged to the federal award.
Determine which investigators are members of the Downstate workforce, based on Step 4 and review the Flow Chart in Step 5A to determine when Individual Investigator Agreements (IIA) or IRB Reliance Agreements (IRA) is required, for investigators who rely on a reviewing IRB other than the IRB of their own institution (e.g, External Investigator relying on the Downstate IRB, Downstate Investigator relying on an External Reviewing IRB).
Note: External investigators must follow the policies of their own institution. When a multi-site research project meets the criteria for Exempt Review, multiple IRBs are used to review the research at each site (e.g., each IRB with jurisdiction over their site's investigators reviews their research), as described in Step 5C2 below.
- An Individual Investigator Agreement (IIA) (click link for form) may be executed between an external Investigator and the Downstate IRB, for non-exempt
human research.
- Typically, the investigator is at an institution without an IRB and/or acts independently from the institution (e.g., moonlighting resident, physician in private practice), or their institution or IRB allows an IIA.
- An IRB Reliance Agreement (IRA) is set up between the Institution and the reviewing IRB.
- When research is conducted by Investigators who are NOT members of the Downstate workforce
and reviewed by the Downstate IRB, an IRA is generally required between the Downstate IRB and the external Institution,
where the Investigator(s) is/are an employee or agent. Alternatively, an IIA may be established with the Investigator(s) in certain situations,
as described above.
- The Downstate IRB has existing IRAs to serve as the reviewing IRB for all research conducted by Investigators from UPB and HHC, Kings County Hospital. Note: These institutions may also use other IRBs, based on their policies.
- The Downstate IRB has an existing IRA with Maimonides Medical Center to review research
conducted by Maimonides investigators under the following conditions:
- PI is a member of the Downstate workforce, AND
- Research is NOT federally funded, NOT industry sponsored, and NOT exempt.
- An IRA may be executed between other external institutions and the Downstate IRB, to review NON-EXEMPT research, when the external institution is a HIPAA Covered entity, and has ALL of the following in place:
- Federal Wide Assurance (FWA) on file with the Department of Health and Human Services, Office of Human Research Protections (OHRP),
- PHS/NIH Financial Conflict of Interest compliant policy,
- A local IRB, Human Research Protection Program (HRPP), or office to confirm local research requirements, AND
- A compliance (audit or QA) program.
- When research is conducted by Investigators from the Downstate workforce and reviewed
by a external IRB (e.g., sIRB, Central IRB, Commercial IRB), an IRA is generally required between Downstate
and the external reviewing IRB.
-
- Downstate has existing IRAs with the following IRBs:
- New IRAs may be established as needed with other qualified IRBs. To be qualified,
the IRB must have at least one of the following criteria:
- AAHRPP Accreditation,
- CARE-Q Certification,
- Member of the SMART IRB Reliance System, or
- Successfully completed a quality assessment within the last 5 years (e.g., OHRP or FDA audit, self-assessment, peer review assessment). Documentation must be provided upon request.
-
- When research is conducted by Investigators who are NOT members of the Downstate workforce
and reviewed by the Downstate IRB, an IRA is generally required between the Downstate IRB and the external Institution,
where the Investigator(s) is/are an employee or agent. Alternatively, an IIA may be established with the Investigator(s) in certain situations,
as described above.
- IRAs and IIAs are processed by the Downstate IRB, following the specific steps below:
- Confirm the external investigator's research cannot be overseen by another IRB (e.g., IRB at the institution where they are an agent or employee, Central IRB, sIRB, etc).
- Request the external investigator complete an Individual Investigator Agreement (IIA) and sign the form digitally. For information on how to complete and sign a fillable PDF, see "TIP 2" at the towards the top of this Electronic IRB Submission webpage. The form must be completed properly or it will be sent back to the investigator to be corrected.
- Send the completed form to the Executive Director, Human Research Protections and Quality Assurance for review and execution.
- The fully executed form will be returned to the external investigator, the study team, and OCAS.
- OCAS will provide instructions on how to complete the Downstate HIPAA training.
- The external investigator must complete the Downstate IRB training requirements outlined in Step 6.
- The executed agreement must be submitted with the Downstate IRB application materials at the time of initial review or when amending a study to add the investigator to research, previously approved by the IRB.
- Consult the Flow Chart in Step 5A to confirm the Downstate IRB can review the research.
- Consult Step 5B to confirm the external site is eligible to execute the agreement.
- Confirm with the external site they are willing to execute an IRA.
- Email the Executive Director of the Downstate IRB to request an IRB Reliance Agreement (IRA) be executed and include the following
information with the request:
- Protocol title and funding information, when applicable.
- Contact information for:
- the Overall (main) PI of study,
- the Downstate Local PI, if not the Overall PI.
- the person responsible for executing the agreement at the external site(s)
- If available, provide:
- FWA#(s) and IORG#(s) for the external site(s)
- Downstate IRBNet #(s)
- Draft protocol(s)
- Draft informed consent document(s)
- Local context (research requirements) of the site(s) seeking IRB approval.
- Conflict of interest adjudication and COI Management Plans of investigators, when applicable.
- Confirmation that all training requirements are met by the external investigators by their sites.
1. Obtain an e-mail or other documentation from the sponsor to indicate review is required by the Reviewing (external) IRB. Save this documentation to include with the Downstate IRB submission during the review of local research context and activation of the study at Downstate, otherwise plan to use the WCG IRB.
2. Contact the Reviewing IRB for a quote for their fee schedule and their specific submission instructions.
Note: The contact for the Advarra IRB, Advarra IRB is: institutions@advarra.com
3. Use the SMART IRB Online Reliance System to establish the IRA:
Go to the SMART IRB Online Reliance System and click “Online Reliance System” at the top of the page.
- Click the blue “Log In” button on the right. If you don’t yet have access to the system, request investigator access by clicking “Request Investigator Access” beneath the “Log In” button. This request will be sent to the Downstate IRB representative for approval.
- Once logged in, the PI/study coordinator/site contact should enter study information into SMART IRB and request the Reviewing IRB (e.g., Advarra) to review the study. This request is emailed to the Downstate IRB’s SMART IRB administrative contact. Note: This step does not require the PI to have Access to the system. A study coordinator may enter the PI information in the system.
- The Reviewing IRB and Downstate IRB will go through a series of online steps to establish the reliance.
- Once the request is approved by the Downstate IRB, the Reviewing IRB will receive a request to review the specific study. Note: During this process, the Downstate IRB Office will provide External IRB with the latest local research requirements. This and other Downstate IRB guidance for Investigators is available at the Downstate IRB Policy and Guidance website.
- An email is generated and sent to all parties indicating that the Reviewing IRB has agreed to review the study. Note: The final agreement and associated documents may be downloaded from the system by all parties.
- Contact the IRB that will oversee the study to
- Confirm the SMART IRB Online Reliance System cannot be used to establish an IRA.
- Confirm the IRB meets the Downstate requirements: To be qualified, the IRB must have
at least one of the following criteria:
- AAHRPP Accreditation,
- CARE-Q Certification,
- Member of the SMART IRB Reliance System, or
- Successfully completed a quality assessment within the last 5 years (e.g., OHRP or FDA audit, self-assessment, peer review assessment). Documentation must be provided upon request.
- Request instructions on how to establish the IRA. Follow the Reviewing IRB's process for establishing the IRA. A paper based, IReX, or other electronic reliance agreement may be established when they are not a member of SMART IRB.
- Provide the above information via e-mail to the Executive Director of the Downstate IRB and also include the following information with the request:
- Protocol title and funding information, when applicable.
- Contact information for the Overall (main) PI of study, institution's IRB or HRPP, and Downstate local PI.
- If available, provide the OHRP FWA# IRBOrg#, IRB# for the Reviewing IRB and Downstate IRBNet #, along with copies the protocol, informed consent document, Reviewing IRB process.
NOTES:
- The IRB Reliance Agreement with the Reviewing IRB and Downstate covers Investigators from the Downstate workforce. Additional IRAs must be established by all other investigators with their own institution.
-
Exceptions to the above process may apply to Tribal and non-U.S. IRBs.
- Review the Flow Chart in Step A to confirm the Downstate IRB has jurisdiction over the research.
- Review Step B to confirm the Downstate IRB may review IRB applications for external investigators or employees and execute any necessary IIA or IRA agreements.
- External investigators should contact their institution to determine their institutional requirements for review of human research.
- Be sure to describe the data sharing between sites and security protections within the Downstate IRB application materials.
- Ideally, identifiable data should not shared between sites; however, identifiable data could be shared when HIPAA and data security protections are in place, applicable disclosures are provided to participants, institutions agree to sharing identifiable the data, and applicable agreements are executed.
- External investigators covered by another IRB and consultants not conducting research should not be included on the Downstate Exempt IRB application; however, details of data sharing between sites must be included, as noted above.
- Refer to the sections below for additional information based on the type of review:
This section under development.
Multiple IRBs may be used for research which qualifies for exempt review, regardless of source of funding.
- In general, an external IRB (typically the IRB of the external investigator's site) reviews the external investigator's research activity for exempt research and MUST review an external investigator who would be designated by the Downstate PI to be an 'investigator for the purposes of COI'.
- The Downstate IRB reviews the Downstate workforce and may also approve other investigators with an IIA (those who are not designated as 'investigator for the purposes of COI').
This section under development.
CAUTION: This process is for the Downstate workforce only. Other investigators must follow the process of the External IRB and their institution. For example, when collaborating with Kings County, the Facility Research Coordinator at Kings County Hospital should provide their local research context to the external IRB.
- Determine whether a Pre-Activation (Pre-Review) by the Downstate IRB is required by the PI, Sponsor, or External IRB, particularly if the Downstate IRB Office needs to perform an administrative review of the language in the informed consent or HIPAA authorization, or other materials to ensure Downstate requirements. The Downstate IRB office will also check for training and COI requirements during the pre-review process.
- Submit the materials to the Downstate IRB in IRBNet. Request a pre-review using Form 11-A3 (Application for IRB Oversight) and check Option B in Section 3 of the form. Be sure to include all relevant materials in the IRB application to conduct the Pre-Review.
- The Downstate IRB Office will issue a Pre-Activation letter after conducting an administrative review and list any recommendations or requirements.
WCG / WIRB CONTACTS:
Client Services:
855-818-2289
If needed, contact Glori Schmeckpeper at gschmeckpeper@wcgclinical.com
PROCESS:
- Plan for WCG IRB fees in the research budget, if applicable (e.g., sIRB fees when Downstate is the prime awardee, IRB fees that are not directly invoiced to an Industry sponsor) and Downstate IRB fee for local review, if applicable (e.g., for Industry Sponsored research). See: Fee Schedule
- Obtain a quote for IRB fees, when applicable (e.g., sIRB fees when Downstate is the
prime awardee, IRB fees that are not directly invoiced to an Industry sponsor).
- To obtain a WCG IRB quote for sIRB fees for specific study, please complete the WCG Single IRB Quote Request Formand forward to Charlie Eibeler at WIRB and Executive Director of the Downstate IRB.
- To obtain information regarding the Downstate discount rate for WCG IRB fees for industry sponsors please contact Charlie Eibeler at WIRB or the Executive Director of the Downstate IRB.
- The WCG IRB will directly bill the industry sponsor. If the sponsor cannot be directly invoiced, WCG IRB will invoice the PI for which SPA will impose an additional 25% processing fee.
- For sIRB studies, WCG IRB will invoice the PI which will be charged to the federal award.
- Sponsored Programs Administration (SPA) will build the IRB fees into the award agreement.
- Complete Pre-Review, if applicable (see step 5D1 above).
- Follow any submission instructions from the WCG IRB, when ready to submit to WCGIRB
- WCG IRB Submission Requirements:
- Initial Review Submission PDF Smart Form
- Protocol/Grant (final/clean copy)
- Consent Form(s) (Word Version) (if applicable)
- Be sure to include the SUNY RF Payment Consent form, when payments to participants is $600 or more in a calendar year or more than $100 per study visit.
- Protocol level Recruitment/Subject material (if applicable)
- Medical License and CV of Principal Investigator (if not on file at WIRB and applicable to the research type). A CV will suffice if the PI is not an MD.
- Site level recruitment/Subject materials (if applicable)
-
Submission Requirements for New Protocol WIRB Approval:
-
If WIRB has already reviewed the research, then you only need to submit the following:
- Initial Review Submission PDF Smart Form
- Consent Form(s) (Word Version) (if applicable)
- Medical License and CV of Principal Investigator (if not on file at WIRB and applicable to the research type). A CV will suffice if the PI is not an MD.
- Site level recruitment/Subject materials (if applicable)
-
Submission Forms are on the WCGIRB website under the “How to Submit” > "Download IRB Forms".
-
Submit documents to through online submission portal:
-
Connexus submission instructions for New Protocol:
- Sign up for an account online then click on the following:
- Make Submission
- Initial Review Submission
- Review of a New Research Protocol
- Upload documents and submit
- Connexus submission instructions if WCG has already reviewed the research:
- Contact WCG to request access to the Study workspace
- Click on “My Studies” and select the Protocol #
- Select “Submit New Investigator”, upload documents and submit
- Obtain approval from the WCG IRB.
- Request activation by the Downstate IRB (see Step 5E7 below).
BRANY CONTACTS:
This section under development.
PROCESS:
This section under development.
ADVARRA CONTACTS:
Contact institutions@advarra.com for Advarra’s fee schedule or to ask any questions.
Advarra resources:
PROCESS:
- Plan for Advarra IRB fees in the research budget, if applicable (e.g., if IRB does not invoice sponsor directly) and Downstate IRB fee for local review, if applicable (e.g., for Industry Sponsored research). See: Fee Schedule
- Complete Pre-Review, if applicable (see step 5D1 above).
- Follow any submission instructions from the Advarra IRB, when ready to submit to Advarra,
please visit cirbi.net
- Select either the investigator application or protocol application, depending on what you wish to submit to Advarra.
- Follow the Advarra CIRB Quick Steps to Register for a CIRB account.
- Follow the Advarra CIRB Quick Steps to submit an Initial Protocol Application.
- Submit all required documents for Advarra IRB review.
- Be sure to include the SUNY RF Payment Consent form, when payments to participants is $600 or more in a calendar year or more than $100 per study visit.
- For any questions on how to submit or to learn more please contact the Advarra Institutions Team at: institutions@advarra.com
- Obtain approval from the Advarra IRB.
- Request activation by the Downstate IRB (see Step 5D7 below).
This section under development.
This section under development.
- After the (external) Reviewing IRB approves the study, submit Form 11-A3 (Application for IRB Oversight) to the Downstate IRB in IRBNet to confirm all local requirements and to activate Downstate as a site. Include the IRB approval letter obtained from the Reviewing IRB and the documents listed in Item C (Section 3) of Form 11-A3. NOTE: Do not include investigators who are not members of the Downstate workforce.
- Describe the data sharing between sites and security protections within the Downstate IRB application materials.
- The Downstate IRB Office will issue a Activation letter after conducting an administrative review and confirming all local requirements are met. The Downstate IRB will stamp any approved consent materials if the materials are not stamped by the external IRB when these materials require stamping under Downstate IRB-01 policy.
- Note: If the Downstate IRB has additional requirements or recommendations after the external IRB approves the study, the PI will need to submit an amendment to the external IRB for their approval. Once amended, include the approved amendment materials to the Downstate IRB, so that Downstate may be activated as a site.
The IRB, OCAS, and COI Committee may consider proposals for alternative IRB and COI review. Contact the Executive Director of the Downstate IRB if an alternative approach is proposed.
GENERAL INFORMATION:
- The Downstate IRB facilitates the process for agreements required in the research and may be requested to review the agreements for congruency with the IRB application materials. The IRB does not approve or execute the agreements. An overview of the process for execution of required agreements is described at the end of this section.
- Establish a Data Use Agreement (DUA) for activities involving limited data sets. Note: If SUNY Downstate is the data holder and is disclosing a limited data set to an external recipient, our DUA template must be utilized. Downstate will only consider the review of another facility's template if we are obtaining the data from them.
- Establish a Business Use Agreement (BAA) when the study involves a non-Downstate workforce member who performs or assists in the performance of a function or activity involving the use, receipt or disclosure of protected health information, unless they are approved as an external investigator by the Downstate IRB when they are covered by a fully executed Individual Investigator Agreement or IRB Reliance Agreement.
- Implement Confidentiality Agreements (CA) when required by the IRB or Sponsor, particularly if needed to address any data security requirements. This is typically required for NIH funded studies. Include the Confidentiality Agreement with the IRB submission.
- Establish a Clinical Trial Agreement (CTA) or Facilities Use Agreement (FUA) when required by Sponsored Programs Administration or an external site. NOTE: DO NOT include the CTA or FUA with the IRB submission.
- Establish a Materials Transfer Agreement (MTA) when required by the Office of Innovation and Partnerships (I&P).
- CAUTION: Research activities covered by an agreement may not begin until any required agreement is fully executed by those with the authority to execute the agreement. Members of the study team are NOT authorized to sign any agreement on behalf of the SUNY RF or SUNY Downstate.
Note: In general, all data shared with external investigators under a multi-site study approved by an external IRB should be de-identified or coded in a manner where the key to the code is only available to investigators who are members of the Downstate workforce. This activity must be approved by the external IRB and subject to local site activation by the Downstate IRB.
EXECUTION OF AGREEMENTS FOR NON-FUNDED RESEARCH:
Send the agreement(s) to Shoshana Milstein, Associate VP, Compliance and Audit and Downstate Privacy Officer. Include any related materials associated with the request. Additional consultation with General Counsel may be required. In general, members of the study team may NOT sign the agreement, unless otherwise instructed by Ms. Milstein or General Counsel. When the agreements are finalized by the designated signatory official, they will be returned to the PI.
Include the FULLY EXECUTED AGREEMENT(S) in IRBNet with the IRB submission; however, unsigned drafts many be included if not yet fully executed.
EXECUTION OF AGREEMENTS FOR FUNDED RESEARCH:
Send the agreement(s) to Ernest Purefied, Director, Contracts with a copy to Sharon Sealy, Executive Director, Deputy Operations Manager. Include any related materials associated with the request. Mr. Denny will review the documentation and prepare it for RF signature. Additional consultation with General Counsel and/or the Downstate Privacy Officer may be required. Members of the study team may NOT sign the agreement. Executed agreements will be signed by the designated signatory official and returned to the PI.
Include the FULLY EXECUTED AGREEMENT(S) in IRBNet with the IRB submission; however, unsigned drafts many be included if not yet fully executed.
Quick Links (referenced in the sub-steps below):
- Guidance on Training Requirements
- Collaborative Institutional Training Initiative (CITI) website
- Guidance on COI Requirements
- For COI disclosure and COI training requirements, the PI determines who on the research
team is an “Investigator for the Purposes of COI,” as defined in the Research Conflict of Interest Policy (RFDMC-01): The project director, Principal Investigator, co-Principal Investigator, personnel
who are considered to be essential to work performance or any other person, regardless
of title or position, who is responsible for the design, conduct or reporting of research.
The PI is responsible for identifying all Investigators involved in their research
activities. If the role of an individual is unclear and that individual is listed
as an Investigator, compliance with all training and filing requirements will be expected.
- Transient staff and trainees, such as medical students, residents and fellows, who may recruit patients and/or collect and handle data under supervision, but are not key to the design, conduct or reporting of research are not considered Investigators for purposes of COI. In addition, staff or trainees who merely implement a protocol developed by an Investigator or enter data into an electronic data capturing system are also not considered Investigators for purposes of COI. However, if a medical student, resident and/or fellow is applying for a research grant, s/he is considered an investigator for COI purposes and, therefore, must complete COI requirements.
- COI disclosures are required by HHC for Kings County investigators who are considered to be “Covered Individuals” as defined by HHC Policy 180-9. A “Covered Individual” means Principal Investigators, Sub-investigators, Collaborators, Consultants, and other Key Research personnel responsible for the design, conduct, or reporting of the research.
- COI disclosures do not apply to other External Investigators, UNLESS the study has
a PHS Award, as defined in the Downstate COI policy.
- PHS Award(s)- Any grant, contract, award, or sub-award including SBIR/STTR Phase II applicants/awardees, but not Phase I SBIR/STTRs, issued or awarded by the United States Public Health Service and its agencies: Agency for Healthcare Research and Quality; Agency for Toxic Substances and Disease Registry; Centers for Disease Control and Prevention; Food and Drug Administration; Health Resources and Services Administration; Indian Health Service; National Institutes Health; and the Substance Abuse and Mental Health Services Administration; and their sub-agencies.
- External Investigators should contact their employer to determine if they have any additional COI disclosure requirements.
Each investigator (or key personnel) must complete the designated training as outlined in the table in the Guidance on Training Requirements.
Creating a CITI account:
1. Register online for Collaborative Institutional Training Initiative (CITI) training
Note: If you have an existing CITI account from another institution, you do not need to create a new one; however, you must affiliate with SUNY Downstate in order to complete the SUNY Downstate requirements and for the IRB to check your training. Please use your Downstate e-mail account so that your training information can be imported into the electronic IRB application and reporting system in the future.
2. Affiliate your account with SUNY DOWNSTATE.
3. Choose either one of groups of basic training modules:
- Group 1: Biomedical Investigators and Key Personnel, Basic Course
- Group 2: Social / Behavioral Investigators and Key Personnel
4. Sign up for supplemental (optional) training (e.g., HIPAA, COI, GCP, etc.) as desired or required, AFTER completing the basic training modules. For investigators who are not members of the Downstate workforce, please complete the optional CITI HIPAA training (module #14) offered through affiliation with SUNY Downstate.
Refresher training is required every 4 years for all training except GCP. GCP training is valid for 3 years.
Enrolling in optional training modules in CITI Program:
Follow this guidance once the basic or refresher modules have been completed.
- Log into CITI.
- On the top section of the site, click on "Courses"
- Scroll down to “Institutional Courses” and select “View Courses” under SUNY-Downstate Medical Center.
CAUTION: If you are from another institution and have not yet affiliated with SUNY Downstate, please do so by clicking the link to “Add Institutional Affiliation”
- Scroll down to the bottom section: “Learner Tools for SUNY - Downstate Medical Center”, then click on, "Add a course".
- This will take you to the page where you select your curriculum.
- Note- There are 14 questions in total on this page.
- Question # 1, 6, 12, and 13 are the only questions that require an answer. All other
questions are optional, so skip the optional training questions that do not apply
to your needs.
- For Question 1, select, "I am not involved in human subjects research" if you have already completed Group 1 or Group 2 courses.
- If you have not already completed one of these courses, please select one of the two groups to complete.
- Be sure to complete the following questions, to take the corresponding optional training:
- Answer Q3 if you are an IO or IRB Chair
- Answer Q3 if you plan to conduct animal research
- Answer Q5 to complete GCP training. The Downstate IRB will accept any module.
- Answer Q6 to complete COI training
- This is not required by the Downstate workforce, as a different course outside of CITI is required.
- Non-Downstate workforce may complete the optional CITI Conflict of Interest training module (various modules are available including #488 and #681) offered through affiliation with SUNY Downstate. Either of the following are acceptable: Group 1: Biomedical Investigators and Key Personnel (COI) or Group 2.Social / Behavioral Investigators and Key Personnel (COI)
- Answer Q7 to complete GCP refresher training. The Downstate IRB will accept any module.
- Answer Q8 to complete clinical research coordinator (CRC) training.
- Answer Q9 to complete Biosafety/Biosecurity training.
- Answer Q10 to complete Export Compliance training.
- Answer Q11 to complete IRB Administration training.
- Answer Q12 to complete Clinical Trial Billing Compliance training.
- Answer Q12 to complete training in GDPR & Human Subjects in the US.
- Answer Q14 to complete training in Protocol Registration and Results Summary Disclosure in Clinical.Trials.gov.
- After all questions/selections are made click, “submit”. You are now enrolled in the course(s) you selected.
- Scroll down and see the section that reads: “Courses Ready to Begin”. Select, “Start Now” to start completing your selected course.
For help, contact Nakih Gonzales at nakih.gonzales@downstate.edu
Downstate IRB Webinars:
We are thrilled to invite you to our expert-led webinar series designed to elevate your IRB application process. These sessions, hosted by the Executive Director of the IRB and IRB Staff, are tailored to provide you with valuable insights, practical tips, and the latest updates in IRB guidelines. Our goal is to help you navigate the complexities of IRB approvals efficiently and effectively.
Please plan to attend these informative webinars and encourage research investigators and coordinators in your area to join as well. Not only will these sessions maximize your success in obtaining IRB approval, but they also offer access to valuable resources and recordings post-event.
Can't attend live? No problem! Register now to secure your spot and receive exclusive access to the webinar recordings. We're here to guide you every step of the way in streamlining your IRB application process.
We look forward to your participation!
- Applications for IRB Determinations
- Recording Date: Jan 17, 2024
- Focus: This session will cover tips and guidance on how to request "IRB Determinations" of Not Research, Not Human Research, or Institution Not Engaged in Human Research.
- Click here to view the recorded webinar. Passcode: $&u6U.2P
- Exempt IRB Applications
- Recording Date: Jan 31, 2024
- Focus: This session will cover tips and guidance on how to obtain approval of research that qualifies for "Exempt Review," such as research projects involving Educational Practices, Educational Tests, Surveys, Interviews, Focus Groups, Observations of Public Behavior, Benign Behavioral Interventions with Adults, and Secondary Use of Information or Specimens (including medical chart reviews).
- Click here to view the recorded webinar. Passcode: .J0NRh1#
- Expedited IRB Applications
- Date: Feb 14, 2024
- Focus: This session covered tips and guidance on how to obtain approval of research that is no greater than minimal risk and qualifies for "Expedited Review," such as clinical studies on FDA approved drugs and medical devices, collection of blood samples, prospective collection of biological specimens by noninvasive means, and collection of data through non-invasive procedures.
- Click here to view the webinar Passcode: b?8Zma7^
Past Presentations:
- IRB Session for the Downstate Clinical Trials Symposium.
- This presentation covers: a) Using an External IRB b) Applicable Clinical Trials and PRS reporting c) What's new with the IRB.
- Click here for the YouTube Video
- Click here for the slides.
- IRB Overview and Updates
- IRB Submission Process:
- New Staff: IRB Orientation (You Tube Video)
- IRBNet - New Project Submission
Biostatistics Presentations
John Conley Division of Medical Ethics and Humanities:
- FREE audio podcast presentations:
- Go to the iTunes Store
- Enter "SUNY Downstate" in the search bar
- Follow links for John Conley Division.
Office for Human Research Protections:
The U.S. Office for Human Research Protections (OHRP) offers various training options to help understand Health and Human Services Regulations. Click on the links below for additional information:
FDA Educational Materials:
The U.S. Food and Drug Administration (FDA) has several web resources for educational materials, some of which are described below:
- Clinical Investigator Training Course
- Clinical Trials Educational Materials
- Comparison of FDA and HHS Human Subject Protection Regulations
- Expanded Access (Compassionate Use): Information for Physicians
- Medical Device Workshops and Conferences
- Search for FDA Guidance Documents
NIH Educational Materials:
- Human Subjects Protections Training
- Research and Training
- Office of Behavioral and Sciences Research Training
External Educational Materials:
The following links are provided for educational materials and resources from external IRBs and other sites.
- Advarra IRB
- Association for the Accreditation of Human Research Protection Programs (AAHRPP)
- Axiom Research Compliance
- BRANY IRB
- Clinical Trials Methodology Course (CTMC)
- Clinical Trials Transformation Initiative
- Complion Resources
- EMORY University IRB
- Kinetiq Knowledge Center
- National Cancer Institute Central IRB
- National Institutes of Health Virtual Seminar on Program Funding and Grants Administration (Track A includes OHRP session on Simplifying Informed Consent)
- Neuro Next
- PI Program: Professionalism & Integrity in Research
- Public Responsibility in Medicine and Research (PRIM&R)
- Quorum Knowledge Center
- SMART IRB
- University of Washington - Research Ethics Training for Health in Indigenous Communities
- WIRB Copernicus Group Webinars
- FORTE Educational Clinical Research Resources
Anyone determined to be an “Investigator for the Purposes of COI” must complete their required COI disclosures as outlined in the Guidance on COI Disclosures and Training Requirements.
Quick Links (referenced in the sub-steps below):
- Downstate Protocol Template (for Downstate Investigator-Initiated research that involves a clinical intervention
- Downstate Protocol Template (With Guidance) - use for non clinical intervention and exempt research.
- Downstate Protocol Template (Shell Only) - use for non clinical intervention and exempt research.
- NIH/FDA Phase 2 or 3 Clinical Trial Protocol template with guidance
- NIH Electronic Protocol Writing Tool
- NIH Behavioral and Social Clinical Trials Template
- Form 7b: Application for Ancillary Review of Unfunded Clinical Research
- Research budget
- CMRC Review Request Form
- CMRC Process
- CMRC Reviewer and Certification Form
- CMRC mailbox
For sponsored projects use the protocol provided by the sponsor.
For non-sponsored projects, please follow the guidance and instructions in one of the following templates as applicable to the research:
- Downstate Protocol Template (for Downstate Investigator-Initiated research that involves a clinical intervention).
- Downstate Protocol Template (With Guidance) - use for non clinical intervention and exempt research.
- Downstate Protocol Template (Shell Only) - use for non clinical intervention and exempt research.
- NIH/FDA Phase 2 or 3 Clinical Trial Protocol template with guidance.
- NIH Electronic Protocol Writing Tool.
- NIH Behavioral and Social Clinical Trials Template.
Unfunded (Downstate support only) Clinical Investigations and Clinical Research
SUNY Downstate Health Sciences University (DHSU) requires ancillary review by the University Hospital at Downstate (UHD) and/or Downstate Health Physicians (DHP, formally UPB) for certain unfunded (Downstate support only) clinical research before IRB approval:
- Medical device trials that require an IDE.
- Drug or biologic trials that require an IND.
- Clinical trials deemed more than minimal risk.
- Clinical research involving bedside infusions, pregnant women, fetuses, children, or cognitively impaired adults.
- Clinical research that prospectively enrolls UHD or DHP patients, or utilizes UHD or DHP resources, unless the sole interactions or interventions are surveys or obtaining consent.
Clinical research of the above types, funded and supported by UHD or DHP, necessitates the respective approval of each organization.
Note: Click here for IRB guidance on the definitions of Clinical Investigations and Clinical Research.
Evaluation Procedure:
Please submit the following with the IRB submission:
- A cover letter detailing costs and resources supported by Downstate (e.g., labor, equipment, space, supplies) and indicating costs covered by University Hospital at Downstate (UHD), Downstate Health Physicians (DHP), and Downstate College/School. Include the % effort and estimated labor cost for each Downstate paid investigator and Downstate paid research staff, excluding residents/fellows. This cover letter must be signed by the PI, Department Chair/Dean, and Department/College Administrator. If the PI is a Department Chair, it must also be signed by the Dean.
- Form 7b: Application for Ancillary Review of Unfunded Clinical Research (signed by the PI)
- The research budget must encompass all expenses. You may use the Research budget template , create your own, or include all necessary details in the cover letter. The budget must thoroughly outline all research costs and the sources of financial support.
Ensure all documents are consistent with study materials. Inaccurate forms will be returned for correction and may delay the review process.
The IRB will share information with the UHD and other relevant departments, including DHP if applicable. The IRB strives to expedite this review process. We appreciate your cooperation during this process. Please send any feedback or questions to IRB@downstate.edu.
Once the study is approved by the ancillary reviewers, the IRB will notify the PI.
NOTE: The pilot forms and budget template are currently undergoing updates and improvements, with the release date yet to be determined. If you prefer to wait, inform the IRB, which will notify you when the forms are available online.
The Central Methodology Review Committee (CMRC) has been appointed by the Downstate Institutional Official on behalf of the Downstate President to certify certain human research protocols meet acceptable standards for study methods, research design, and statistical analysis. The types of protocols reviewed by the CMRC are limited to those outlined on the CMRC Request Form.
The CMRC must certify the protocol before it is submitted to the Downstate IRB.
For current submissions, the types of protocols reviewed by the CMRC are limited to those outlined on page 2 of the CMRC Review Request Form.
Click on the links below for more information:
- CMRC Membership Roster
- CMRC Consultants
- CMRC Review Request Form
- CMRC Process (includes FAQs and instructions for completing e-forms in Adobe Reader)
- CMRC Reviewer and Certification Form
NOTE: The Departmental and College level Scientific Review Committee (SRC) is NOT required by Downstate IRB for any study undergoing CMRC review, including those reviewed during the pilot phase. SRC review may be valuable for other types of studies and may or may not be required by the Department Chair or Dean. The PI should consult with the Department Chair or Dean if they have any questions about their requirements for SRC review.
Questions about the CMRC may be sent to the CMRC mailbox.
See Tip 5 G (above) to include the proper project description.
Review guidance for:
Develop the informed consent templates and related materials or request a waiver, when applicable:
Note: Informed consent is not a requirement for exempt research applications; however, HIPAA authorizations (or waivers) apply to exempt research involving PHI.
- 8-1: Simple Version Informed Consent Template
- 8-2: All-In-One Version Informed Consent Template
- 8-3: HIPAA Research Authorization
- 8-4: HIPAA Authorization for Psychotherapy Notes
- 8-5: Electronic Information Sheet with HIPAA Authorization for e-survey, e-interview, or e-focus group
- 8-6: Electronic Information Sheet (NO PHI / NO SIGNATURES) for e-survey, e-interview, or e-focus group
- 8-7: Information Sheet with HIPAA Authorization
- 8-8: Information Sheet (NO PHI / NO SIGNATURES)
- 8-9: Pregnancy Follow-Up Consent
- 8-12: SUNY RF Payment Consent Required for research payments of either 1) $600 or more per calendar year or 2) more
than $100 per study visit.
- IRS Form W-9 (Use with above form, but do not include with IRB submission)
- IRS Form W-8BEN (Use with above form, but do not include with IRB submission)
- 8-13: Assent Form (for age 7-12 years)
- Research Subject Recruitment Authorization Forms: These forms may be used to obtain contact information for patient recruitment through their clinicians. Download in PDF format and click "Highlight Existing Fields" in upper right corner to edit. To use these forms refer to Policy HIPAA-28 Use and Disclosure for Research Purposes
- 8-17: NYS Medical Release Form. This form is accepted by UHD and other providers and can be used when researchers need to request medical records of their participants from other sites.
- 8-18: HIPAA Waiver.
THE PI MUST E-SIGN THE HIPAA WAIVER USING ADOBE SOFTWARE.
A HIPAA waiver is required for retrospective review of PHI (i.e., medical records review, lab reports).
A HIPAA waiver is required for review of PHI for recruitment purposes, when the investigators are not the healthcare providers of the potential participants.
- 8-19: Waiver of Informed Consent Requirements. Required when waiving documentation (e.g., signature) of informed consent, the entire process of informed consent, or required elements of informed consent.
NOTE: If PHI is involved, the IRB will accept the HIPAA waiver form (Form 8-18) to document a request a waiver of informed consent.
- 8-20: HIPAA Authorization Form. This form may be used to obtain authorization for review of medical records or PHI for case reports, when a journal requires informed consent be obtained from patients. This is a patient request form and can be used, for example to request patient information for each patient in a case report or case series.
- 8-21: Authorization for Release of Health Information to News Media and to the General Public (Media HIPAA Authorization Form). This form must be used when PHI, including potentially identifiable video or photos, will be obtained to be used by news media, including professional medical or healthcare journals, professional meetings, symposiums, poster sessions, or other events. This form is also required when a single patient case study is conducted about an extremely rare disorder, because sharing information about a single patient with an extremely rare disorder would make them identifiable.
Note: If video or photos will be obtained for any marketing purposes, please contact the Office of Communications & Marketing for additional guidance.
A short form written informed consent form stating that the required elements of informed consent required have been presented orally to the research participant or their legally authorized representative, and that the required key information was presented first, before other information, if any, was provided. The IRB shall approve a written summary (this can be the English version of the consent or a separate written summary) what is to be said to the research participant or the legally authorized representative. When this method is used, there shall be a witness to the oral presentation.
Refer to IRB Guidance: Obtaining Legally Effective Informed Consent and HIPAA Research Authorization to determine if the Short Forms should be used for the study.
CAUTION: In general, written translation of the long form is expected over the use of the short-form process when the research anticipates the enrollment of five or more research participants with limited English proficiency of the same language (e.g., 6 Spanish speaking participants), for the following types of research:
- Phase 0, 1,1/2, 2, 2a, 2b, or 2/3 Clinical trials which are determined to be greater than minimal risk without any anticipated therapeutic benefit for the research participants
- Studies which are determined to be a minor increase over minimal risk, when there is no direct benefit to the research participant;
- Complex clinical trials; or
- When required by the sponsor.
Short Form Templates:
Note: Use the versions immediately below for research that must comply with the 2018 version of the Common Rule (e.g., research consents which contain a "Key Information" section)
- Short Form (Arabic)
- Short Form (Simplified Chinese)
- Short Form (Traditional Chinese)
- Short Form (English)
- Short Form (Haitian Creole)
- Short Form (Russian)
- Short Form (Spanish)
Note: Below are the translation certificates for the short forms immediately above this section. These do not need to be submitted to the IRB, but are here to be saved to the research record or to be shared with a sponsor.
- Translation Certificate for Short Form (Arabic)
- Translation Certificate for Short Form (Simplified Chinese)
- Translation Certificate for Short Form (Traditional Chinese)
- Translation Certificate for Short Form (Haitian Creole)
- Translation Certificate for Short Form (Russian)
- Translation Certificate for Short Form (Spanish)
Note: Use the versions immediately below for research previously approved research grandfathered under the pre-2018 Common Rule (e.g,. initially approved prior to January 21, 2019 or for newer research which is not subject to the Common Rule (e.g., FDA or HIPAA regulated research that is not Federally funded that use research consent forms that do not require the "key information" section.)
- Short Form (Arabic)
- Short Form (Simplified Chinese)
- Short Form (Traditional Chinese)
- Short Form (English)
- Short Form (Haitian Creole)
- Short Form (Russian)
- Short Form (Spanish)
Note: Below are the translation certificates for the short forms immediately above this section. These do not need to be submitted to the IRB, but are here to be saved to the research record or to be shared with a sponsor.
- Translation Certificate for Short Form (Arabic)
- Translation Certificate for Short Form (Simplified Chinese)
- Translation Certificate for Short Form (Traditional Chinese)
- Translation Certificate for Short Form (Haitian Creole)
- Translation Certificate for Short Form (Russian)
- Translation Certificate for Short Form (Spanish)
Effective on February 20, 2020, the IRB requires the PI to submit a current CV or NIH Bio-sketch with each new study submitted to the IRB.
The following additional materials are required, when applicable to the study:
- Recruitment Materials, including, but not limited to:
- fliers
- brochures
- ads
- e-mails
- telephone or verbal script
- web site materials
- social media postings - NOTE: Any Downstate representation on social media must be
approved by the
the Office of Communications and Marketing. The IRB will forward the ad to them for approval after conducting a preliminary review.
- Questionnaires or Surveys.
- REDCap: Research Data Capture and Analysis System
- Note: REDCap Surveys are HIPAA compliant and can be used to capture Protected Healthcare Information (PHI).
- Qualtrics Survey Software
- Note: Do not use Qualtrics for surveys that must capture Protected Health Information (PHI).
- If you already have an account, click here to log into the Downstate-licensed Qualtrics system.
- REDCap: Research Data Capture and Analysis System
- Data Collection Tools (or list of data to be collected).
- Clinical Investigations involving an IND:
- Investigator Brochure (IB)
- FDA Form 1572 NOTE: For FDA regulated clinical investigations involving an IND, the Clinical Investigator (PI) must be listed on the FDA Form 1572, which is sent to the Sponsor. Sub-Investigators (co-investigators) must be listed on the FDA Form 1572. Key Personnel do not need to be listed on the FDA Form 1572. A copy of the signed FDA Form 1572 must be submitted to the Downstate IRB at the time of initial review and when submitting an amendment to change the Clinical Investigator or Sub-Investigators.
- IND letter from FDA or sponsor
- When applicable (e.g., when there is no IB), please include the following items to
help facilitate IRB review:
- Published literature about the chemistry, manufacturing, and control of the drug substance and product;
- A summary of previous human experience with the drug product;
- Sufficient information regarding the source, purity, quality, and method of preparation and delivery of the drug used in the research;
- Information regarding the pharmacology and toxicity of the drug product in animals.
- Clinical Investigations to evaluate the safety and effectiveness of an implantable
investigational medical device:
- Device Package Insert
- IDE Letter or SR/NSR determination from the FDA or sponsor
- When requested by the IRB, provide Credentials of study staff performing clinical interventions.
- When requested by the IRB, provide the contract or agreement with the Sponsor.
- IRB may need to confirm consistency of informed consent language regarding injuries, additional costs, EU GDPR disclosures, data security requirements, or other information.
- HIPAA Preparatory to Research Certification Form
MOST COMMONLY USED FORMS:
- Form 11-A1: Application for Exempt Review
- Form 11-A2: Application for Expedited or Full Review
- Form 11-A3: Application for External IRB Oversight
- Form 11-A4: Application for Determination Letter (IRB Decision Aid) for "Not Research, Not Human Research, or Institution Not Engaged"
- Form 11-4C: Checklist for Acknowledgment of a SELF-DETERMINATION of "Not Research" or "Not Human Research" and Attestation of Institutional Compliance
- FORM 11-A4Q: Application for an IRB Determination of "NOT Research" or "NOT Human Research" for a Quality Improvement, Quality Assurance, Performance Improvement, or Evidence Based Practice Activity.
- Form 11-10: Application For Downstate Workforce Activation of Exempt Research or IRB Determinations (Not Research, Not Human Research, or Downstate Not Engaged) Approved by an (External) Reviewing IRB
LESS COMMONLY USED FORMS:
- Form 11-A1B: Application for Exempt Review - DOJ/DIJ Funded Research Only
- Form 11-A5: Application for Expanded Access to Investigational Drug/Biologic for Treatment Use
- Form 11-A6: Application for HUD for Clinical Purposes
- Form A7: Application for Independent Honest Broker Assurance Agreement
- Form 11-8: Exclusion of Pregnant People and/or Plans to Study Outcomes of Unexpected Pregnancies
- Form 11-9: Research involving Pregnant People and/or Fetuses
See Item 11A (below) for guidance on which application form should be used.
MOST COMMONLY USED FORMS:
Exempt Review:
Use "Form 11-A1: Application for Exempt Review".
The details of the exemption categories are described on the IRB application form. In general, use the exempt form for non-DOJ funded research that includes the following types of research:
- Normal educational practices in established educational settings
- Educational tests, surveys, interviews, or observation of public behavior
- Benign behavioral interventions with adults with prospective agreement
- Secondary research for which consent is not required (includes retrospective chart reviews with HIPAA waiver)
- Federal research and demonstration projects
- Taste and food quality evaluation and consumer acceptance studies
Note: Use Form 11-A1B: Application for Exempt Review of DOJ/DIJ Funded Research Only.
Expedited or Full Review
Use "Form 11-A2: Application for Expedited or Full Review".
Use Form 2 for any research which meets the criteria for expedited review or full board review. Examples of non-exempt research which may meet the criteria for expedited review, when they are no greater than minimal risk included the following:
- Clinical studies of drugs and medical devices only under specific conditions (no IND or IDE)
- Collection of blood samples
- Biological specimens obtained by non-invasive means
- Collection of data through non-invasive means
The following types of research must be reviewed by the full board:
- Studies involving greater than minimal risk
- Clinical Trials involving IND, IDE, or HUD
- If referred by the expedited reviewer
- Initial review of research that meets the criteria for “expedited review” category
#1 or #2:
- If it involves biomedical research interventions with children, pregnant people, neonates, prisoners, or cognitively impaired adults
Note: The term “biomedical research intervention” is interpreted to be an intervention that involves the research of a drug, biologic, and/or medical device as the object(s) of the study. This term is not meant to be interpreted as a medical procedure, such as a blood draw or the use of an FDA approved medical device to measure what the device is intended to measure. Nor is it intended to include the use of an FDA approved/licensed agent that is used per clinical indications in a clinic nor the review of medical records about an agent. All other criteria for expedited review must apply for the study to be reviewed by expedited review.
External IRB Oversight:
Use "Form 11-A3: Application for External IRB Oversight".
In general, the PI can request the use of an external IRB for multi-site studies and when the research is approved by a Tribal IRB.
IRB Decision Aids (IRB Determinations)
There are multiple forms that can be used to request an IRB determination that IRB oversight is not required for activities that are classified as Not Research, Not Human Research, or for Downstate Not Engaged. The form that should be used depends on the applicable situation:
- Form 11-4C: This is the easiest form to submit an IRB acknowledgment of a SELF-DETERMINATION by the Project Lead.
- This form must be completed for ALL non-human research and all non-research projects or activities carried out by residents and may also be used by students or other members of the Downstate workforce if required by their Department or College/School.
- If you are not sure of the determination, it is best to use 11-A4Q and should also be used when a journal requires an official IRB determination rather than an acknowledgement of a self-determination.
- Form 11-A4Q: This is the easiest form to use for an official IRB DETERMINATION for a "Not Research" or "Not Human Research" determination, typically used to for a QI, QA, PI, or EBPA that does not meet the regulatory requirements for IRB Oversight. This form is typically used by DNP Students in the College of Nursing but may be used by any student or member of the Downstate workforce.
- Form 11-10: ONLY use this form if an external reviewing IRB (not Downstate) makes the determination.
This form is submitted the Downstate IRB to activate the project at Downstate. It
is used when the external reviewing IRB issues a determination of Not Research, Not
Human Research or Downstate Not Engaged. It can also be used if the external IRB
approves human research as Exempt under the Common Rule.
- Do NOT use this form for human research activities that qualify for Expedited or Full Board review.
- This form is typically used when an investigator has a joint role or appointment with Downstate or when a Downstate student or trainee is conducting activities at their employer's institution and seeks approval by their institution's IRB or HRPP for multi-site activities which includes Downstate
- Downstate does NOT require an IRB Reliance Agreement to activate exemptions or determinations submitted with this application form; however, the (external) reviewing IRB may require one.
- This is considered an administrative review to confirm Downstate requirements and avoids duplicate IRB review process.
- Before using this form, the study team must confirm the (external) reviewing IRB is willing to approve the activities conducted by the Downstate workforce.
- Form 11-A4: This form is all encompassing and may be used to request an IRB determination that
IRB oversight is not required for activities that are classified as Not Research,
Not Human Research, or for Downstate Not Engaged.
- It is the most complex form and should only be used if the others won't work for your situation.
- It is generally required for a Not Engaged determination unless an external reviewing IRB makes such as determination as noted in Form 11-10.
LESS COMMONLY USED FORMS:
The following forms are required when enrolling or excluding pregnant people, or for studies which require contraception, pregnancy testing, or for studies that may involve or impact partners of participants who become pregnant:
- Form 11-8: Submit this form when excluding Pregnant People and/or when there are plans to study outcomes of unexpected pregnancies
- Form 11-9: Submit this form when the research involves Pregnant People and/or Fetuses
Exempt Research conducted or supported by Department of Justice:
Use "Form A1B: Application for Exempt Review - DOJ/DIJ Funded Research Only" when applicable, based on funding.
Expanded Access:
Use "Form A5: Application for IRB Approval of Expanded Access to Investigational Drug/Biologic for Treatment Use"
The terms expanded access, compassionate use, pre-approval access, access, and treatment use are used interchangeably to refer to an investigational drug/biologic when the primary purpose is to diagnose, monitor, or treat a disease or condition rather than obtain any information that is generally derived from clinical trials (e.g., safety or effectiveness data).
Clinical Use of a HUD (HDE):
Use "Form A6: Application for HUD for Clinical Purposes"
A Humanitarian Use Device (HUD) is a medical device intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or manifests in fewer than 4,000 individuals in the United States per year. A physician may request approval to use a HUD for clinical purposes.
For additional guidance see the FDA guidance for IRB review of a HUD; however, be aware that this FDA guidance is under review to be in accordance with the 21st Century Cure’s Act.
NOTE: There is a distinction between “clinical use” (non-research use) of a HUD and “investigational use/clinical investigation” of a HUD. If a HUD will be used for a clinical investigation (e.g., safety and effectiveness data is collected for a FDA Pre-market Approval), a full board IRB application must be completed and reviewed under an HDE if it is SR device study. An informed consent document is always required for a clinical investigation of a HUD/HDE.
Honest Broker:
Form A7: Application for Independent Honest Broker Assurance Agreement
An independent honest broker is a Downstate employee who has access to desired research data or specimens by virtue of his or her responsibilities as a member of the workforce providing the coded data or coded specimens for a project but who is not a member of the research team. An honest broker CANNOT serve as investigator or key personnel on the same project, because (s)he has access to the key to the code and can identify the participants. The Downstate IRB does not permit the use of this agreement with business associates or individuals who are not members of the Downstate workforce.
An honest broker can access protected health information and provide an investigator with coded data, de-identified data or a limited data set, once approved by the IRB. Submit this form with an IRB Decision Aid or IRB application, as applicable to the project.
Contact the IRB office if you are not sure which form to use.
Refer to IRB Submission Tip #2 towards the top of this web page for details.
Click here for direct link for IRBNet.
The Downstate IRB & Privacy Board uses IRBNet for the electronic submissions and management of human research activities and required reporting.
Anyone associated with Downstate may create an IRBNet user name and password by following the instructions below:
Notes:
- Each user agrees to comply with the IRBNet Individual User Terms of Use.
- All members of the Downstate workforce MUST use their Downstate e-mail address when setting up their account.
Step 1: Create an IRBNet user account
- Go to www.irbnet.org and click the “New User Registration” link. Follow the online instructions. Complete all items with red asterisk (*).When asked to identify your “organization” type SUNY in the text box and then select “SUNY Downstate Health Sciences University, Brooklyn, NY”.
- Remember to click on the “Register” button in order to finalize your “New User Registration.”
- Press the “Continue” button on the “Registration is Complete” page and follow “Step 2” to activate your IRBNet user account.
Step 2: Activate your IRBNet user account
- After successful completion of “Step 1,” the User will receive an activation e-mail to the registered e-mail address.
- Sign-in to the e-mail account that you entered into the system and click on the link within that e-mail to activate your IRBNet account.
- You may begin using IRBNet as soon as activation is complete.
NOTE: To request a forgotten password enter IRBNet Username or e-mail address here.
OPTIONAL: If desired, the submission may be shared with an IRB Administrator to request and obtain their pre-review for any additional feedback.
Please refer to the guidance for the IRBNet (IRB Application and Reporting System) for more details on how to use the system.
Quick Links:
- Form 13.1 Scientific Review Committees (SRC) Roster
- Form 13.2 Scientific Review Committee (SRC) Review Form
NOTE: The Departmental and College level Scientific Review Committee (SRC) is NOT required by Downstate IRB for any study undergoing CMRC review (see step 7 above). SRC review may be valuable for other types of studies and may or may not be required by the Department Chair or Dean. The PI should consult with the Department Chair or Dean if they have any questions about their requirements for SRC review.
Unless CMRC review (see step 7 above) is completed, Scientific/Scholarly Review (SR) is required prior to IRB approval of the following types of research projects:
- Application for Expedited or Full Board Review (Form 11-A2), when the study is not eligible for expedited review, which are typically studies which are greater than minimal risk
- Application for Expedited or Full Board Review (Form 11-A2), when the study is eligible for expedited review under categories (1A) or (1B) (e.g., studies involving a drug, biologic, or medical device for which neither an IND or IDE are required).
Unless otherwise required by the Department Chair or Dean, the IRB does NOT require Downstate SR review for submissions for the following applications:
- Application for Exempt Review (Form 11-A1)
- Application for which qualify for expedited Review (Form 11-A2), unless the research reviewed under categories (1A) or (1B), as noted above
- Application for External IRB Oversight (Form 11-A3), unless local Scientific Review is required by the external IRB
- Application for Determination Letter (IRB Decision Aid) for "Not Research, Not Human Research, or Institution Not Engaged" (Form 11-A4)
- Application For Downstate Workforce Activation of Exempt Research or IRB Determinations (Not Research, Not Human Research, or Downstate Not Engaged) Approved by an (External) Reviewing IRB (Form 11-10)
- Application for Humanitarian Use Device (HUD) for Clinical Purposes (Form 11-A6)
- Application for Expanded Access to Investigational Drug/Biologic for Treatment Use (Form 11-A5)
The Scientific Review Committee (SRC) is a scholarly and scientific review committee charged with reviewing research at Downstate. The purpose of the SRC is to evaluate the quality of the proposed activity when required by the IRB, Department Chair, Dean, or PI to ensure the research meets an acceptable standard of scientific rigor to improve the likelihood of yielding valid and meaningful information.
Please share the IRBNet submission with the SRC member to obtain their review and approval via e-signature. To determine which SRC member should review the research, please see: Form 13.1 Scientific Review Committees (SRC) Roster
Form 13.2 Scientific Review Committee (SRC) Review Form is completed by an SRC reviewer and must be attached to the submission in IRBNet. The SRC member is no longer required to e-sign the submission in IRBNet when the SRC review PDF fillable form is signed electronically.
Ancillary reviews by various departments or committees may be required as an administrative process for Downstate policy or at the discretion of the IRB for any human research protection concern. Examples of Ancillary Reviews may include Scientific Review Committee (SRC) (see above), Institutional Biosafety Committee (IBC), Radiation Safety, Radiology, Pharmacy, and UHD Pathology.
To determine if an ancillary review is required prior to IRB approval of a specific project, other than noted below, please consult the IRB website, contact the IRB, or refer to the policies of the above committees or departments. The IRB requires the following ancillary reviews in advance of granting IRB approval for the specific conditions noted below:
Ancillary review by UHD Pathology is required when the research involves any of the following:
- Services or assistance of the UHD Pathology Laboratories (Clinical Laboratory, Histology Lab and/or Surgical Pathology).
- Prospective collection of fresh specimens that require processing by UHD Pathology Laboratories, including tissue, blood and fluids (see UHD Exempt Tissue Policy: “LAB3 Human Tissue Fluid and Foreign Matter Exempt From Submission for Pathology Examination.”
If uncertain about the need for Pathology Review, please e-mail Dr. Anna Plourde for a determination. If she states Pathology Ancillary review is not required, attach the e-mail determination to the IRB submission.
Step 1:
- Refer to the UHD Pathology Instructions, Forms, and Fees posted on the UHD Pathology Research Services web site.
- Complete and submit “Step 1 Form: Preparation for Use of UHD Laboratory/Patient specimens for Research Projects: Clinical, Histology, and Surgical Pathology Labs Feasibility Determination” to Pathology.
Step 2:
- Complete and submit the IRB application after the UHD Pathology Laboratories approves the feasibility of using their services to obtain IRB approval.
- When submitting the IRB application in IRBNet, please share the IRBNet submission with the pathology representative so that (s)he may e-sign the submission. E-signature is required before the IRB can grant final approval.
Step 3: After IRB and Biosafety (if needed) approvals are granted, complete and submit the “Step 3 Form: Protocol of UHD Laboratory Use/Patient specimens for Research Projects: Clinical, Histology, and Surgical Pathology Labs. Your pathology approval number will be then assigned.
Caution: If any changes are required after final IRB approval, an amendment must be submitted to the IRB.
UHD pathology approval is required prior to IRB approval when patient material obtained for research will affect the clinical care of a patient (e.g., if the research proposes a surgical sample is divided for research and clinical purposes), as determined on a case by case basis by either the IRB or UHD Pathology.
Note: UHD pathology approval may be required for other activities, prior to starting the research. Refer to UHD policy or IRB guidance on the IRB application or IRB website for additional details.
NIH NExTRAC review is required for any study that involves human gene therapy or any deliberate transfer of recombinant or synthetic nucleic acid molecules, or DNA or RNA derived from recombinant or synthetic nucleic acid molecules into one or more human research participants.
Ancillary review by the IBC is required when the research involves any of the following:
- Work on human-derived biological materials at Downstate which does not take place in a CLIA certified lab
- Packaging and shipping of human-derived biological materials at Downstate
- Hazardous substances
- Infectious agents
- Recombinant or synthetic nucleic acid molecules
- Any study that requires NIH NExTRAC review
For more information, please visit the IBC web site or contact Ms. Lydia Bailey at the IBC Office at (718) 270-3912 or IBC@downstate.edu.
Note: IBC approval may be required for other activities, prior to starting the research. Refer to IBC policy or IRB guidance on the IRB application or IRB website for additional details.
All Downstate studies involving a drug or biologic must be approved by the Downstate Research Pharmacy.
- Please share this IRB Submission with the Research Pharmacist or designate, so that (s)he may review the application and electronically sign the submission.
- For questions regarding Research Pharmacy review, contact Motria Mishko at (718) 270-4340 or motria.mishko@downstate.edu
- If Motria is not available, please consult with Stanley Moy, PharmD, BCPS, BCIDP, at (718) 270-4121 or Stanley.Moy@downstate.edu
Downstate Pharmacy approval is required prior to IRB approval for clinical investigations at Downstate that involve an IND.
Note: Downstate Pharmacy reviews for other studies involving a drug, including biologic can take place after IRB approval; however, cannot start until the Research Pharmacy approves the study. Refer to Downstate Pharmacy policy or IRB guidance on the IRB application or IRB website for additional details.
The IRB may require other types of ancillary review when the IRB determines there is a human research protection concern that requires the ancillary review. Examples may include review by the following groups:
- Radiology or Radiation Safety (i.e., for concerns with the risk or level of radiation exposure of a research participant).
- Biostatician (i.e., for concerns with possible design flaws or concerns with the data analysis plan).
- Consultant (i.e,, when there is not sufficient expertise on the IRB to evaluate the study).
- Privacy Officer or Data Security Officer (i.e., to address any concerns with HIPAA regulations, GDPR regulations, privacy, confidentiality, or information security).
- Ethics Consult.
- IBC (i.e. for any biosafety concern when IBC review is not otherwise required).
- Biomedical engineering (i.e., to review equipment used in the research, if there is a safety concern or unknown risk of using the equipment).
- Office of Regulatory Affairs.
- Office of General Counsel.
When an ancillary review is required by Downstate or another institution, but NOT required as a condition for Downstate IRB approval, the investigator must document approval of any pending required ancillary review within the research record prior to starting the research or enrolling any research participants, as applicable. The IRB may provide a copy of the approval letter to the corresponding department or committee that should complete the ancillary review. If an ancillary reviewer requires or recommends any modifications to the previously IRB approved research, any such modification must be subsequently approved through an IRB amendment, prior to implementation of changes. If desired, by the PI or sponsor, the PI may submit a copy of the documentation of ancillary approval not requiring any modifications to the IRB for the IRB to acknowledge; however, this is not a requirement of this policy.
The Downstate Department Chair of Dean must review and approve the following types of applications, prior to IRB approval:
- IRB Applications for Exempt review, Expedited review, Full Board review, or External IRB Oversight
- Application for a HUD for Clinical Purposes
- Application for Expanded Access to Investigational Drug/Biologic for treatment
If the research impacts multiple Departments or Colleges, each area where the research takes place needs to approve the study.
Please share the submission with the applicable Department Chair(s) or Dean(s) and request their electronic signatures in IRBNet.
A Department Chair or Dean may delegate this authority to another person through the Delegation of e-Signature Form.
Within IRBNet, click on the “Submit this Package” button on the left menu and follow the instructions.
Note: Once the IRB Application package is submitted it is locked. If any changes are needed to the package, contact the IRB to request the package be unlocked.
The IRB process is iterative in nature. The IRB submission is automatically withdrawn if response is not timely; however, PI the may request more time if needed.
There are two types of revisions.
- The IRB will pre-review the package and may unlock it to request minor revisions via
expedited review.
- Revise the package as requested and lock package and mark revisions complete.
- The IRB will require the submission of a revised package when major modifications
are required.
- Submit a follow-up package in IRBNet.
- Include a point-by-point response cover letter.
NYC Health + Hospitals, Kings County (KC) Requirements:
- Follow policies of both KC and Downstate.
- All research conducted at KC or by KC investigators must follow NYC H+H policies,
including:
- Requirements of Operating Procedure No: 180-9
- Obtain approval in "System to Track and Approve Research" (STAR) prior to conducting the research at NYC. Note: Downstate IRB Approval is required before information can be entered in STAR. For more information refer to the NYC H+H, Kings County policies on the IRB policy tab.
- Copies of all consent forms involving registered patients enrolled in any study must be filed with the Electronic Medical Record.
- The Site (local) Principal Investigator for a study conducted at KC must be a full-time, part-time or voluntary physician who is a member of the Medical Staff at Kings County and who has appropriate clinical privileges as defined in the Facility's Medical Staff Bylaws. This individual must also be approved by the reviewing IRB as personnel on the study.
- The Director of Research for KC is Dr. Shahriar Zehtabchi. He must e-sign all KC research submissions prior to the Downstate IRB approving the submission. Questions may be directed to: Shahriar.Zehtabchi@nychhc.org
- All submissions that involve Artificial Intelligence must undergo an ancillary review process at KC and STAR approval cannot be granted until this process is complete. Please submit a copy of the research protocol to Craig White who will start this process in parallel with the IRB review and approval process. The AI ancillary review will be a part of the KC STAR approval process in addition to IRB requirements.
- When sharing PHI between sites, the sites must execute a data sharing agreement. This process takes place during the KC STAR review and approval process.
- All IRB submissions that involve KC must be shared in IRBNet with the following individuals
so that have access to the submission through the life of the study:
- Dr. Shahriar Zehtabchi
- Nicholas Bove, MPH
- Craig White
- For any questions about NYC H+H, Kings County policy, please contact:
-
Nicholas Bove, MPH, Associate Director of Research by email boven@nychhc.org or phone: 718-245-3466
- Craig White, Senior Clinical Research Coordinator; Email: whitec12@nychhc.org; Phone: 718-245-3502
- Please copy Bryce Petty, Bryce.Petty@nychhc.org on all email correspondence.
-
If other NYC H+H investigators are included in the research with Downstate investigators, the research will require review by the BRANY IRB and the Downstate IRB. For specific guidance, see Step 5 (above) or contact the Downstate IRB.
Other External Sites:
- Follow policies of both the external site and the Downstate IRB
- Follow requirements of any agreement with Downstate, including an IRB Reliance Agreement and/or an Individual Investigator Agreement, BAA, MTA, DUA, etc.
- Upon approval by the IRB, the study team should do the following:
- Review the IRB approval letter for accuracy and appropriate determinations.
- Check the approval date and expiration date in letter and approved documents (consent, recruitment materials, etc.). An expiration date is NOT needed for recruitment materials
- Contact the IRB if there are any discrepancies, errors, or questions.
- Share applicable documents with sponsor.
- File documents in study binder.
- Ensure the study meets Applicable Clinical Trial Requirements (ACT) of the FDA and the ACT website is updated within the required deadlines. The FDA requires Applicable Clinical Trials to be registered within 21 days of enrollment of the first participant. In addition, the International Committee of Medical Journal Editors and other journals require registration of clinical trials prior to enrollment of the first participant.
- Understand and ensure the requirements of sponsor.
- Understand reporting requirements to the IRB, sponsor, and FDA.
- Do not use laboratory reports from research laboratories for diagnosis, treatment and prevention of disease, unless the research laboratory is properly certified or accredited.
- Complete any required ancillary reviews that are still pending approval.
- Finalize clinical trail agreements, facilities use agreements, with Sponsored Programs Administration, as applicable for the study.
Submit the applicable application forms to the IRB after the IRB has granted initial approval:
- Form 20-B1: Application for Acknowledgment
- Form 20-B2A: Application for Amendment
- Form 20-B2B: Application for Amendment - STAFF CHANGES ONLY
- Form 20-B3: Application Form for Reportable Event
- Form 20-B4: Application for Continuing Review/Check-In/Study Closure/Re-Activation
- Log into IRBNet.
- Click on the “My Projects” tab, and then select the study.
- After opening the study, click on “Create a New Package” (last tab under Project Administration). CAUTION: DO NOT CLICK "Create New Project" in error.
- Click on “Designer” tab on left menu.
- To Create a "SUNY Downstate - Registration Form for DMC/IRB Review," do the following:
- On the “Designer” page select “Start a Wizard”
- Select the “SUNY Downstate Registration Form for DMC/IRB Review.”
- You will then be prompted to either create a new wizard from scratch or you may clone an existing wizard. Make a selection and choose “Continue.” With an existing project you will most likely wish to select clone an existing wizard.
- Follow the instruction and click “Next” to move through the document.
- At the end of the form, you must press the “Save and Exit” button.
- If you need to update the existing "SUNY Downstate - Registration Form for DMC/IRB
Review," click on the pencil icon for “SUNY Downstate – Registration Form for DMC/IRB
Review”
- Using the drop down menu at the top of the page, jump to the section that needs to be edited, for example “Project Personnel”
- Edit this section of the form as needed (e.g., for example, when changing research staff click “X” to remove personnel or click “Add Another Individual” at the end of the form.
- Once you are finished filling out the form, you must press the “Save and Exit”. The registration form is automatically saved on the designer page.
- This action will bring you back to the “Designer” page where you can begin to add as many documents as you require for this IRB submission.
- To attach completed applications or documents, click on "Attach New Document" on the Designer page. Attach the required document(s). Be sure to edit the document type for each attachment. Once the document is added, you will be given the option to select the document type.
- To delete a document, click on the "RED X" next to the document on the Designer page.
- To share the submission with the PI or others (e.g, to request e-signature), click
“Share this Project” and follow prompts.
- Once shared, the PI (or others) may e-sign the submission by clicking “Sign this Package” tab.
- Once all signatures are obtained, click “Submit this Package” and follow prompts.
REMINDERS:
-
- If adding staff, please ensure all required training and applicable conflict of interest disclosures have been completed. For more information, please see the IRB Guidance on Training and COI Requirements, in Step 6 (above).
- For information on how to use IRBNet, see Step 12 (above).
- The PI must electronically sign the package.
- Each new PI must e- sign the submission, when adding co-PIs.
- If a PI changes, the Department Chair or Dean must e-sign the submission.
Acknowledgments:
- Submit Form 20-B1: Application for Acknowledgment to request acknowledgment by the IRB (e.g., notices from an external IRB, external reportable events, new training documents, etc.).
Amendments (2 Types):
- Submit Form 20-B2A: Application for Amendment to request approvals for all changes. All amendments must be approved by the IRB before they can be implemented.
- Submit Form 20-B2B: Application for Amendment- STAFF CHANGES ONLY to change study staff. This amendment must be approved by the IRB before any new staff can do research on the study.
Reportable Events:
- Submit Form 20-B3: Application Form for Reportable Event for any reportable event within the required deadline(s) outlined in Policy IRB-01.
Continuing Review/Check-In/Study Closure/ Re-Activation:
Please Note: If the study is completed or only de-identified data is undergoing data analysis, please consider submission of an Application For Final (Study Closure) to the IRB for the purposes of closing the study.
Submit Form 20-B4: Application for Continuing Review/Check-In/Study Closure/Re-Activation
IMPORTANT NOTES:
- Securely retain data in accordance to Policy IRB-01, IRB approved documents, and sponsor requirements. Do not destroy any research data until required retention periods have passed.
- Only include data for sites that have oversight by the Downstate IRB. If this is a multi-site study, do not include data for sites outside of the jurisdiction of the SUNY Downstate IRB.
- If the study is completed or only de-identified data is undergoing data analysis, please consider submission of a final report form to the IRB for the purposes of closing the study.
When seeking continuing review and approval from an external Reviewing IRB, the Principal Investigator (PI) should concurrently submit Form 20-B4 to the Downstate IRB. This practice promotes the concurrent review by the Reviewing IRB and the administrative review of local requirements by the Downstate IRB Office.
Upon receipt and pre-review, the Downstate IRB may either unlock the submission or issue a Conditional Activation letter to the Principal Investigator (PI), which will include a request for a copy of the Reviewing IRB’s approval notice.
Once the PI has provided the Reviewing IRB's approval notice and the Downstate IRB has verified that all local research requirements have been met, the continuing approval of the study will be officially activated.
Overview of Joint Continuing Review and Approval Process:
- Follow the process required by the Reviewing IRB to secure continued IRB approval.
- The PI should submit Form 20-B4 to the Downstate IRB.
- This can be done before receiving the continuing approval notice from the Reviewing IRB.
- Form 20-B4 should not be submitted to the Reviewing IRB.
- Following pre-review of the Downstate submission, the Downstate IRB will either unlock the submission or issue a Conditional Activation letter to the PI.
- After receiving the continuation approval notice from the Reviewing IRB and ensuring all local requirements specified by the Downstate IRB have been met, the PI must update the IRB submission to the Downstate IRB and lock the submission so that the review and activation process may continue.
- The Downstate IRB will activate the study for continuation after the Downstate IRB confirms the Reviewing IRB has issued the continued approval of the study and all local Downstate research requirements have been satisfied.
- Simultaneous compliance with both the Downstate IRB and the Reviewing IRB is essential for an efficient review process, particularly when the Reviewing IRB review might be delayed.
For any queries or assistance, please don't hesitate to contact the IRB Office.
Quick links to Quality Assessment Program (QAP) Guidance and Forms:
- Quality Assessment Program
- Quality Assessment Program -Template Letters to PI
- Form 21-1: Quality Assessment Form
- Form 21-2: Corrective & Preventative Action Plan (CAPA) Form
Overview:
The goal of the Quality Assessment Program (QAP) is to review, inspect and verify the ethical conduct of human research, integrity of data, adherence to the IRB approved protocol, and applicable institutional, state, and federal regulations, policy, and guidance. This program is non-punitive in nature designed to be a productive process for investigators while striving for continuous improvement in every area of the research enterprise. It is important to address newly discovered problems right away and use this as a learning tool to prevent future problems. It is important for investigators and QAP Assessors to be initiative-taking and look for similar issues in other studies. The QAP program is a way to assess readiness for external audits by the Food and Drug Administration (FDA), Office of Human Research Protections (OHRP), State Department of Health (NYSDOH), or a sponsor. With advanced planning and strategic corrective actions, the Downstate QAP improves opportunities for continuous quality improvement and protection of research participants.
Study Team Preparation:
Being prepared for a QAP review is critical to the success of the review. While the PI may receive advance notice for a not-for-cause review, a for-cause review or other audits by the FDA, OHRP, NYSDOH, or sponsor, may occur without notice or very minimal notice. Preparing for such reviews involves daily efforts and requires the study team to follow best practices and always maintain a state of readiness.
For best practices related to clinical trials, please contact the Downstate Clinical Trials Office.
All study and IRB files should be complete, current, and correct. All IRB and research staff should be up to date and current with all required education and training.
Procedures:
Note: For full details, please refer to: Quality Assessment Program
- Research projects are selected for the QAP Assessment.
- In general, Routine (Not-For-Cause) Quality Assessment will occur on a quarterly basis.
- For-Cause Quality Assessments may occur upon approval by the IRB Chair or Vice-Chair.
- The Assessors will conduct quarterly reviews of IRB documentation.
- The Clinical Trials Office (CTO) or Office of Compliance and Audit Services (OCAS) may follow the QAP program to assess the research as determined by their offices.
- Notification of PI:
- The QAP Assessor notifies the PI in advance to schedule a Routine (Not-For-Cause) Quality Assessment.
- For-Cause Quality Assessments may occur without prior notification. Follow-up written notice should occur as the assessment takes place.
- An example of these notices are available at: Quality Assessment Program -Template Letters to PI
- The IRB will notify the PI of any applicable findings during the quarterly reviews of IRB documentation.
- All asessments are completed using Form 21-1: Quality Assessment Form.
- The Assessor and PI sign the form once complete.
- The PI submits the form to the IRB in IRBNet as a follow-up package, for review and acknowledgement.
- When applicable or as determined by the IRB, OCAS, or CTO, the study team completes
Form 21-2: Corrective & Preventative Action Plan (CAPA) Form. The IRB, OCAS, or CTO may provide suggested recommendations for the CAPA, when applicable.
- The CAPA Author and PI sign the form once complete.
- The PI submits the form to the IRB in IRBNet as a follow-up package, for review and acknowledgement.
- When applicable, submit any reportable events discovered during the QAP to the IRB within the required reporting timelines as outlined in Policy IRB-01. See Step 20 for Form 20-B3: Application Form for Reportable Event.
- The QAP Assessment Team may review the response submitted to the IRB and may supply more information; however, the IRB has the final authority over issuing any IRB determination related to the QAP.
Disclaimer: The SUNY Downstate web site includes links to information and sites created and maintained by other public or private organizations. SUNY Downstate provides these links solely for its users’ information and convenience. When users select a link to an external site, they are leaving the SUNY Downstate web site and are subject to the privacy and security policies of the external sites. These sites do not necessarily operate under the same laws, regulations, or policies as SUNY Downstate. SUNY Downstate does not control or guarantee the accuracy, relevance, timeliness, or completeness of information on a linked web site. SUNY Downstate does not endorse the organizations sponsoring linked web sites and does not endorse the views they express or the products and services they offer. SUNY Downstate cannot authorize the use of copyrighted materials contained in linked web sites and is not responsible for transmissions users receive from linked web sites.