UHD Performs first Brooklyn Barostim Implantation for Heart-Failure Patient
By Office of Communications & Marketing | Sep 12, 2022
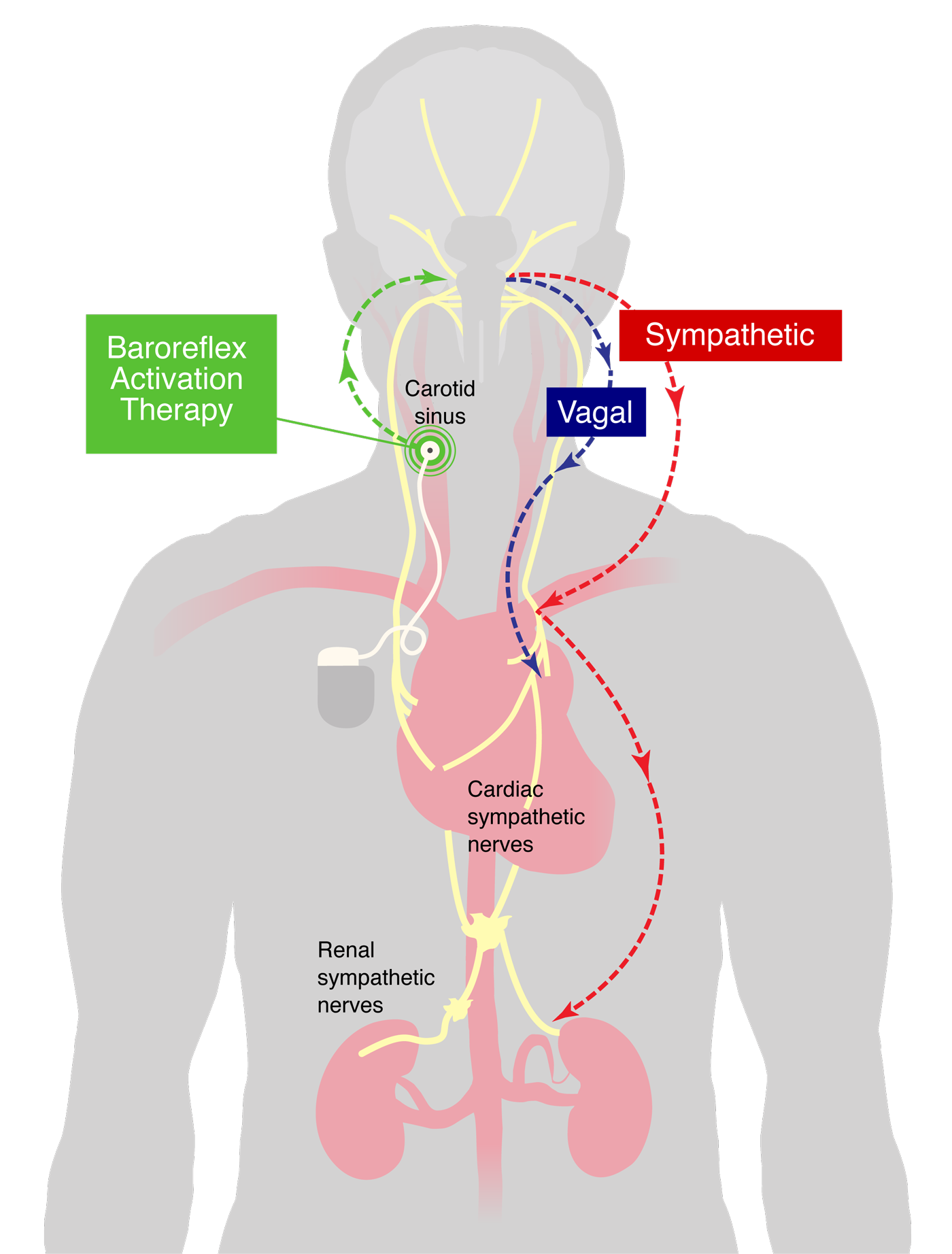 Congratulations to the team of Adam Budzikowski, M.D., Ph.D., Panos Kougias, M.D., and MSc, Marina Svyatets, M.D. who recently completed UHD's—and Brooklyn’s—first successful implantation of the Barostim™ Baroreflex Activation Therapy.
The procedure took place the first week of August at UHD on an 85-year-old patient
with a history of systolic heart failure, permanent atrial fibrillation, implanted
defibrillator, and progressively worsening exertional dyspnea. The patient tolerated
the procedure well and is recovering speedily.
Congratulations to the team of Adam Budzikowski, M.D., Ph.D., Panos Kougias, M.D., and MSc, Marina Svyatets, M.D. who recently completed UHD's—and Brooklyn’s—first successful implantation of the Barostim™ Baroreflex Activation Therapy.
The procedure took place the first week of August at UHD on an 85-year-old patient
with a history of systolic heart failure, permanent atrial fibrillation, implanted
defibrillator, and progressively worsening exertional dyspnea. The patient tolerated
the procedure well and is recovering speedily.
 The world’s first FDA-approved heart failure device to use neuromodulation—the power
of the brain and nervous system—to improve the symptoms of patients with systolic
heart failure, the Barostim Baroreflex Activation Therapy was designed to treat heart
failure patients who have had little to no success with other proven treatment options.
The world’s first FDA-approved heart failure device to use neuromodulation—the power
of the brain and nervous system—to improve the symptoms of patients with systolic
heart failure, the Barostim Baroreflex Activation Therapy was designed to treat heart
failure patients who have had little to no success with other proven treatment options.
Unlike other heart failure treatments, the Barostim neuromodulation device is a minimally-invasive implantable technology therapy for the symptoms of heart failure. The device does not touch the heart; instead, utilizing an electrode on the patient’s carotid artery, electrical impulses are sent to advise the brain of the heart’s condition, allowing its function to improve.
As a result, the heart regains strength over time as heart failure symptoms lessen, allowing patients to resume normal activities. In addition, the therapy is customizable to each patient’s needs. As a result, it can potentially reduce other heart failure-related health risks such as kidney disease, stroke, and death.
We look forward to providing this option as a resource to heart-failure patients who are eligible for this surgery