About Dr. Alfred Stracher: A Life in Research
Alfred Stracher (1931–2013)

Alfred Stracher was born and raised in Albany, New York. He received a B.S. degree in Chemistry at Rensselaer Polytechnic Institute in 1952 and a Ph.D. in Chemistry at Columbia University in 1956. Post-doctoral fellowships sponsored by the National Foundation for Infantile Paralysis followed, the first with Lyman Craig at the Rockefeller Institute for Medical Research (1956 to 1958), the second with K.U. Linderstrøm-Lang at the Carlsberg Laboratory (1958 to 1959). At the time, both institutions were world-renowned centers in protein chemistry. Both institutions produced graduates and fellows who were to become the next generation of leading scientists in the burgeoning field of Biochemistry.
Stracher came to SUNY Downstate Health Sciences University as an Assistant Professor of Biochemistry in 1959. He became Professor of Biochemistry in 1968 and a Distinguished Professor of the State University of New York in 1997. He was chairman of the Biochemistry Department from 1972 to 2006 (the longest serving Biochemistry chair in U.S. medical schools) and Director of the Center for Drug Delivery Research at Downstate Medical Center from 2010 to his death in 2013. Overall, he was an active faculty member at SUNY Downstate Health Sciences University for 54 years.
1959 to 1969: Early Years at Downstate
At Downstate Dr. Stracher began research on rabbit skeletal muscle myosin, a major protein of the contractile process of striated muscle. His work focused on the active site of the myosin ATPase. Using sophisticated sulfide-disulfide exchange methods, he discovered the SH2 site of myosin, a cysteine residue which regulates myosin ATPase activity.
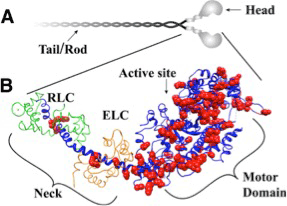
In the mid-1960s the structure of myosin was highly controversial. It was known that myosin could be degraded by trypsin into an insoluble helical fragment (LMM) and a soluble globular fragment (HMM). The latter could be further degraded to a fragment (SF1) that retains the ATPase and actin-binding properties of native myosin. A group at The Johns Hopkins School of Medicine presented strong biophysical evidence, particularly from analytical ultracentrifugation, that myosin comprised 3 long polypeptide chains which formed a 600 kDa structure. A group at Harvard presented strong biochemical evidence that myosin contained just 2 long polypeptide chains. There seemed to be no compromise between the conflicting claims.
Into the fray came the Downstate group of Alfred Stracher and Paul Dreizen, then Assistant Professor of Medicine and fresh from a 3-year NSF fellowship in biophysics at MIT. In a series of incisive experiments using a variety of biochemical and biophysical methods, their group discovered that native myosin also contains smaller proteins (light chains), and established the subunit structure of myosin as two intertwined heavy chains (each approximately 200 kDa) that separated to form a globular head region consisting of 2 SF1 fragments, each containing part of one HC together with 2 different light chains of about 20 kDa. This work, reported in JBC (1966, 1968) and PNAS (1966, 1969), has been seminal to all further work on myosin and the molecular basis of muscle contraction.
1970 to 1989: Middle Years at Downstate
During the 1970's, there was widespread interest in non-muscle myosins and actins from diverse tissues. Stracher played a pioneering role in this work using platelets as a model tissue. His group was among the first to identify actin in the cytoskeletal system as a second form of actin (ABB, 1975) and the role of filamin as an actin-binding protein. He showed that cytoskeletal actin filaments interact directly with platelet membranes, that phosphorylation of the actin-binding protein, filamin, plays a regulatory role in cytoskeletal assembly (BBRC, 1984, 1985), and that calcineurin-dependent dephosphorylation in the C-terminal region of filamin plays a signaling role in connection with platelet activation and aggregation (ABB, 2006). In other work on platelet actomyosin, Stracher showed that short-term storage of platelets is accompanied by extensive proteolysis of myosin in its head region but not in the rod (JCI, 1974).
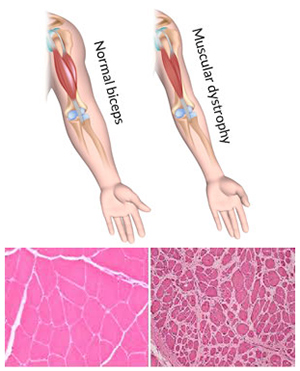
Before the cloning of the dystrophin gene in 1986, it was thought that an abnormal myosin was responsible for Duchenne and Becker muscular dystrophy. Stracher surmised that intracellular proteolysis of an abnormal myosin might cause the degeneration which occurs in dystrophic muscle. Using cell cultures of normal and dystrophic muscle, he confirmed that protease inhibitors such as pepstatin and leupeptin delay degeneration of dystrophic compared with normal muscle (Exp. Neurology, 1976). In work with Julie Rushbrook, using the avian model of muscular dystrophy which resembles human Duchenne dystrophy, he explored whether persistence of an embryonic myosin, a form whose existence was not yet firmly established, might be the putative abnormal myosin. Their work produced definitive evidence for the presence of an embryonic myosin heavy chain during normal chick muscle development (PNAS, 1979). Together with work from France and Australia, this helped set the stage for molecular biology studies of myosin. They later showed that adult muscles in avian dystrophy contained substantial amounts of developmental myosin heavy chains characteristic of normal post-hatch chickens (Cell Motility, 1981; Biochemistry, 1987).
Stracher then treated murine dystrophy successfully with the protease inhibitor leupeptin (PNAS, 1981), suggesting that cellular protease inhibitors might delay the onset of muscle degeneration in human Duchenne dystrophy. In 1986, Stracher was a major force in the establishment of a Protein Sequencing Facility at Downstate for centerwide use.
1990 to 2013: Later Years - The Calpain Hypothesis
Stracher's protease inhibitor approach to disease treatment became even more complex and compelling in the early 1990's. The discovery of ubiquitin and the ubiquitination of misfolded or locally damaged proteins targeting them for selective degradation highlighted degradation in biological and pathological processes. Calpain, a highly selective, non-lysosomal, Ca2+-activated cysteine protease, is especially interesting for its regulatory, rather than direct proteolytic role in this process. Calpain nicks polypeptide chains at a few sites, setting them up for ubiquitination and degradation. Stracher's work on protease inhibitors led to testing calpain inhibitors in a variety of chronic neuromuscular diseases which are categorized by tissue degeneration and atrophy. While leupeptin could be of value in initial studies, there was a need for highly selective peptide inhibitors of calpain and possibly also a drug delivery system to move inhibitors into target tissues at greater concentrations. Attempting to fulfill this goal fully occupied him for the last 15 years of his life. A 1998 Gem Lecture at SUNY Downstate, entitled Nerve and Muscle Degeneration: The Calcium / Calpain Hypothesis described his work. Searching for more selective peptide inhibitors of calpain and a system to deliver inhibitors to the target tissue would occupy Dr. Stracher for the last 15 years of his life.
He organized a large group of investigators across multiple clinical disciplines within SUNY Downstate and elsewhere to test his protease inhibitor concept in animal experiments, initially using leupeptin as the therapeutic agent. Subsequently, in association with Leo Kesner, Stracher developed the novel, blood brain barrier-permeable, calpain inhibitors CYLA (Cys- Leu-Arg), and together with Leo Kesner and Abraham Shulman for ALA-1.0. In a series of papers, these protease inhibitors were shown to be therapeutically effective in animal models of muscular dystrophy, multiple sclerosis, Parkinson's disease, Huntington's disease, cochlear disease, sensorineural hearing loss, tinnitus, traumatic brain injury and post-traumatic epilepsy, and amyotrophic lateral sclerosis (ALS), among other diseases. CYLA has been used successfully in animal experiments on acute and progressive autoimmune encephalitis and transient retinal ischemia. Stracher and Kesner first established the company CepTor, and subsequently ProTor Pharma, for research and development of their protease inhibitors and patented drug delivery systems. Work on CYLA and ALA-1.0 is continuing, with an aim toward progression to clinical trials.
Other Professional Activities and Honors
Alfred Stracher served on the editorial boards of several major journals in the fields of the neuroscience and biochemistry and was the founder and Editor-In-Chief of the journal, Drug Delivery - The Journal of Delivery and Targeting of Therapeutic Agents. He was also a member of several prominent professional societies. He has a long list of honors and awards, including prestigious fellowships from the Commonwealth Fund (1967) and the Guggenheim Foundation (1974), and honorary appointments as Visiting Professor in Biophysics at King's College, University of London, and Visiting Professor of Zoology at Oxford University in 1974.
He was appointed as a Career Scientist of the Health Research Council of the City of New York (1962-1972). From 1981 to 1989, he was a member of the Patent Policy Board of the State University of New York. He was a member of the Drug Task Force of the Muscular Dystrophy Association and served for many years as a member of the Neurological Disorders Study Section (NSP-B) of the National Institutes of Health. In 1984, he was appointed by the Director of NINDS to serve as a member of the Special Review Panel of the Jacob Javits Centers for Excellence in the Neurosciences.

In 1998, he received the Ailanthus Award in recognition of his many years of service to Downstate Medical Center. He was elected to honorary membership in AOA and awarded an honorary MD degree from Downstate Medical Center in 1998. He was Founding President of the Robert Furchgott Society at SUNY Downstate in 2006.
Recently he received an award for work on protease treatment of muscular dystrophy and other neuromuscular diseases from the Technion University in Israel and was elected a Fellow of the Accademia dei Lincei, Italy's highest scientific honor.
Alfred Stracher was an exemplary scientist and a dedicated academician, always optimistic with a generous spirit. His scientific contributions, spanning more than six decades, have profoundly influenced the course of basic and translational research in muscle function and muscle wasting, and the prospective treatment of chronic neurodegenerative diseases.