
Henri Tiedge, PhD
Distinguished Professor
Physiology and Pharmacology, Neurology, Medicine
Regulatory RNAs are important mediators of gene expression in eukaryotic cells. At the center of our research are brain cytoplasmic RNAs (BC RNAs), a subtype of regulatory RNAs that control neuronal gene expression at the level of translation. BC RNAs are targeted to synapses where they regulate the synthesis of local proteins. The mechanism is important for long-term structural and functional modulations of synaptic connections, and thus for the control of neuronal excitability and plasticity.
The dynamic modulation of synaptic connections is a key underpinning of neuronal functionality and plasticity. In our lab, we work to understand how neurons implement such modulation through local translational control of gene expression in synapto-dendritic domains.
The concept of local translational regulation at the synapse addresses a central conundrum of long-term synaptic plasticity: the problem that long-lasting, activity-dependent alterations of synaptic strength depend on changes of gene expression but on the other hand require that such changes be instituted in an input-responsive, locally restricted manner. With local control of gene expression, a synapse acquires independent decision-making authority, an essential prerequisite for the differential modulation of thousands of input-receptive postsynaptic domains.
Local translation at the synapse is modulated by physiological stimuli such as transsynaptic activity and trophic action. Our work is directed at molecular mechanisms that couple the action of such stimuli to the control of local translation. Such control is essential to restrict synaptic protein synthesis to times of demand. Several types of translational regulators have been identified at postsynaptic sites, prominent among them small BC RNAs and the fragile X mental retardation protein (FMRP) (Kindler et al., 2005; Iacoangeli and Tiedge, 2013).
Neuronal BC RNAs are translational repressors that are highly abundant in dendrites and at the synapse. They are targeted to postsynaptic domains by virtue of spatial codes that are contained in noncanonical RNA motif structures (Muslimov et al., 2006, 2011). (Figure 1). BC1 and BC200 RNAs reversibly repress translation by targeting eukaryotic initiation factors 4A and 4B (Wang et al., 2005; Eom et al., 2014). BC RNA translational control rapidly shifts from a repressive to a permissive state upon receptor activation, a switch that is brought about by dephosphorylation of eIF4B, release of BC RNA from the factor, and initiation of translation (Eom et al., 2014).
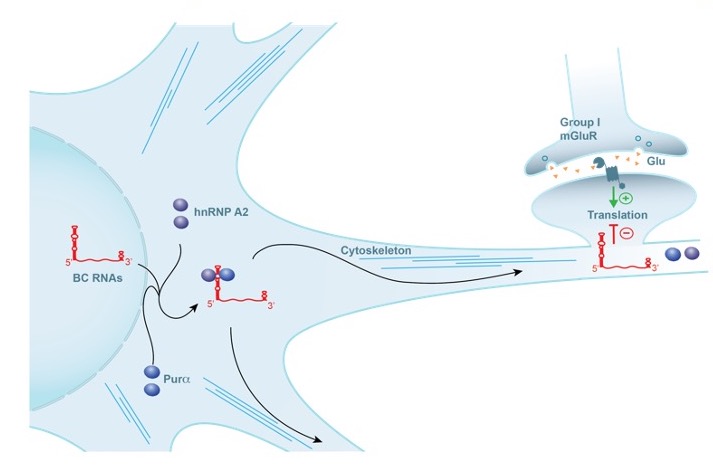
Figure 1. RNA transport and local translation in neurons. BC RNAs are transported to postsynaptic microdomains by RNA transport factors hnRNP A2 and Purα (Muslimov et al., 2022). At the synapse, BC RNAs control translation of local mRNAs, acting as repressors in the basal default state (Eom et al., 2014).
Research with BC1 knockout animals has shown that cell-wide lack of BC1 RNA gives rise to neuronal hyperexcitability, epileptogenic susceptibility, and cognitive dysfunction (Zhong et al., 2009; Iacoangeli et al., 2017). Dysregulated neuronal RNA control can thus be a cause of neurological and cognitive impairment. Near-identical phenotypic alterations were observed when synapto-dendritic targeting of BC1 RNA was inhibited as a result of competition for RNA transport factor hnRNP A2 by expanded CGG-repeat FMR1 mRNA in a mouse model of fragile X premutation disorder (Muslimov et al., 2018). Recent work has shown that autoantibodies from a subset of lupus patients with neuropsychiatric involvement displace transport factors hnRNP A2 and Purα from BC RNA dendritic targeting elements (Muslimov et al., 2019). Synapto-dendritic delivery is thereby inhibited (Figure 2), and resulting lack of the RNAs at synapses causes severe seizure susceptibility (Muslimov et al., 2019, 2022). Seizures can be completely prevented by application of BC RNA decoys (Muslimov et al., 2022), indicating that such decoys may have utility in the treatment of neuropsychiatric lupus. In summary, the combined data indicate that BC RNA intraneuronal transport impairments can result in pathogenicity.
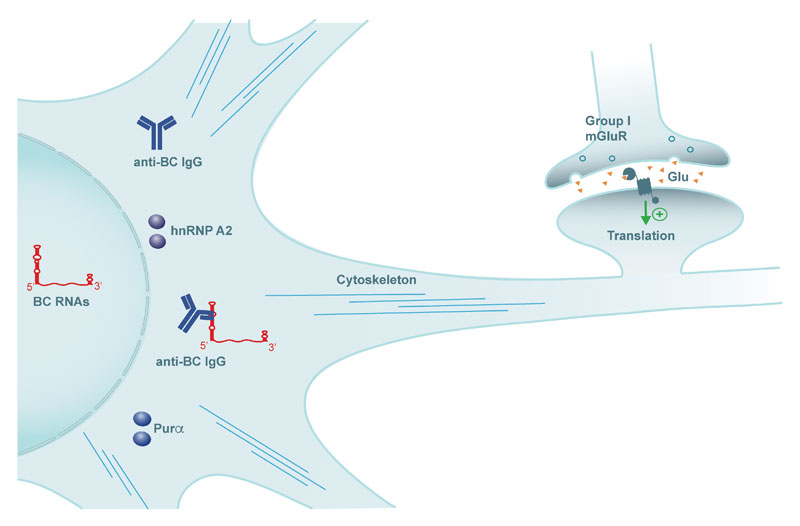
Figure 2. Lupus autoantibodies (anti-BC IgG) target BC RNA structural elements that are requisite for dendritic transport, resulting in synapto-dendritic delivery deficits and phenotypic manifestations (Muslimov et al., 2019, 2022).
Research in Tumor BiologyWe are conducting an independent research project that is aimed at establishing the biological relevance of BC RNAs in tumor cells, and at developing early cancer diagnostic and prognostic tools. Based on our finding that BC RNAs are detectable at significant levels in oocytes and spermatogonia as well as in various types of malignant tumor cells, we suggest that:
(1) BC RNAs play a key role in translational control in germ cells (Muslimov et al., 2002).
(2) Dysregulated BC200 RNA expression results in altered translational control in malignant tumor cells.
Our research is aimed at establishing the role of BC RNAs in translational control mechanisms in tumor cells, its functional relevance in tumorigenesis and tumor progression, and the clinical utility of dysregulated expression in tumor cells. In particular, we will develop the utility of BC200 expression for tumor diagnosis and prognosis (Iacoangeli et al., 2018). This work is covered by a number of patents.
PersonnelValerio Berardi, Ph.D., Research Assistant Professor
Ilham A. Muslimov, M.D., Ph.D., Research Assistant Professor
Annalina Sanfelici, B.S., Medical Student
Nickie Uwoghiren, B.S., Medical Student
Service Functions
Reviewer for various scientific journals and funding organizations
- Wang, H., Iacoangeli, A., Lin, D., Williams, K., Denman, R. B., Hellen, C. U. T., and Tiedge, H. (2005). Dendritic BC1 RNA in translational control mechanisms. J. Cell Biol. 171, 811-821.
- Muslimov, I. A., Iacoangeli, A., Brosius, J., and Tiedge, H. (2006). Spatial codes in dendritic BC1 RNA. J. Cell Biol. 175, 427-439.
- Mus, E., Hof, P. R., and Tiedge, H. (2007). Dendritic BC200 RNA in aging and in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 104, 10679-10684.
- Iacoangeli, A., Rozhdestvensky, T. S., Dolzhanskaya, N., Tournier, B., Schütt, J., Brosius, J., Denman, R. B., Khandjian, E. W., Kindler, S., and Tiedge, H. (2008). On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA 105, 734-739.
- Zhong, J., Chuang, S. C., Bianchi, R., Zhao, W., Lee, H., Fenton, A. A., Wong, R. K., and Tiedge, H. (2009). BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability. J. Neurosci. 29, 9977-9986. Editorial Comment J. Neurosci. 29 page i.
- Muslimov, I. A., Patel, M. V., Rose, A., and Tiedge, H. (2011). Spatial code recognition in neuronal RNA targeting: Role of RNA – hnRNP A2 interactions. J. Cell Biol. 194, 441-457. Highlighted in JCB Editorial Short, B. (2011). RNA targeting gets competitive. J. Cell Biol. 194, 350.
- Muslimov, I .A., Tuzhilin, A., Tang, T. H., Wong, R. K. S., Bianchi, R., and Tiedge, H. (2014). Interactions of noncanonical motifs with hnRNP A2 promote activity-dependent RNA transport in neurons. J. Cell Biol. 205, 493-510. Featured in Biobytes Podcast.
- Eom, T., Muslimov, I. A., Tsokas, P., Berardi, V., Zhong, J., Sacktor, T. C., and Tiedge, H. (2014). Neuronal BC RNAs cooperate with eIF4B to mediate activity-dependent translational control. J. Cell Biol. 207:237-252.
- Muslimov, I. A., Iacoangeli, A., Eom, T., Ruiz, A., Lee, M., Stephenson, S., Ginzler, E. M., and Tiedge, H. (2019). Neuronal BC RNA transport impairments caused by systemic lupus erythematosus autoantibodies. J. Neurosci. 39, 7759-7777. Highlighted in a news release by the Society for Neuroscience.
- Muslimov, I. A., Berardi, V., Stephenson, S., Ginzler, E. M., Hanly, J. G., and Tiedge, H. (2022). Autoimmune RNA dysregulation and seizures: therapeutic prospects in neuropsychiatric lupus. Life Sci. Alliance 5, e202201496. Highlighted in a SUNY news release.