
Sheryl S. Smith, PhD
Distinguished Professor
Physiology and Pharmacology
α4βδ GABAA receptors increase expression at puberty in the mouse hippocampus where they generate a tonic inhibition. Because these receptors express on the dendritic spine adjacent to excitatory synapses, my lab is interested in the role this developmentally-regulated inhibition plays in cognition and synaptic pruning of dendritic spines during adolescence, which impacts behavioral flexibility in adulthood. This receptor is also the target for neurosteroids which alter mood. Adolescence is a vulnerable period for the development and exacerbation of disorders such as anxiety as well as autism and schizophrenia, which are also interests of the lab.
Neuronal circuits in the hippocampus underlie spatial learning and, as part of the limbic system, also play a role in the development of anxiety. My laboratory studies how the activity of these circuits is sculpted by inhibition generated by GABAA receptors, which can then lead to changes in cognition, mood and pathological states such as epilepsy. Our most recent studies focus on changes generated by inhibition during the period of adolescence, a critical period for certain types of learning and when certain neuropsychiatric disorders, such as anxiety and schizophrenia first develop.
Our primary focus is the α4βδ GABAA receptor, a membrane protein which emerges at the onset of puberty on the dendrites and spines of pyramidal cells in the CA1 hippocampus and other CNS areas.
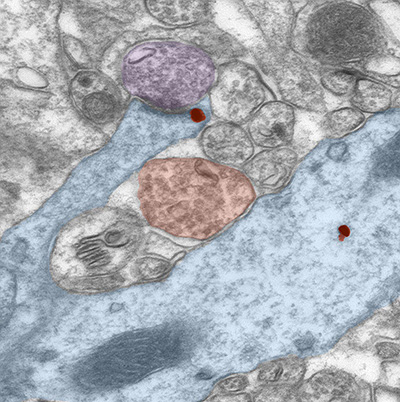
Figure 1. Increased expression of α4βδ GABAA receptors on dendritic spines of CA1 hippocampal pyramidal cells at puberty. The electron microscopic image shows a spine emanating from a dendritic shaft of a pyramidal cell (blue) at puberty. δ subunit labeling (red dot) occurs peri-synaptic to an excitatory synapse on that spine. Glutamatergic (purple) and GABAergic (red) terminals are nearby. (Shen et al., Science 2010)
Inhibition and learning during adolescence
We have shown that the presence of this inhibitory receptor adjacent to excitatory synapses reduces the activation of NMDA receptors, which is the initial step in the learning process. At puberty, spatial learning is impaired as is the induction of long-term potentiation (LTP) in the CA1 hippocampus, considered an in vitro model of learning (Shen et al., Science 2010). These findings may help to explain why the onset of puberty has been shown to represent the end of a critical period for learning certain tasks (such as language acquisition in humans).
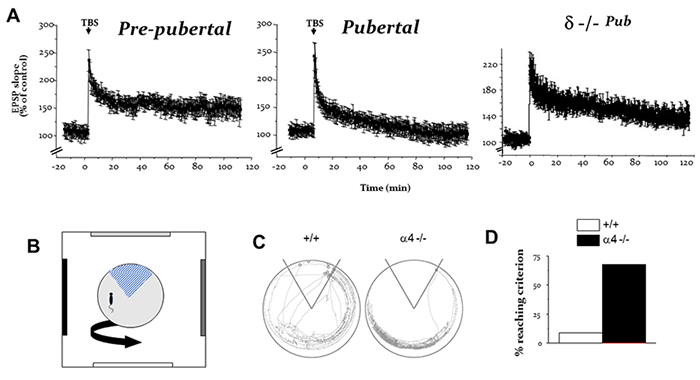
Figure 2. Pubertal expression of α4βδ GABAA receptors impairs synaptic plasticity and spatial learning of female mice. A, Long-term potentiation was achieved with theta burst stimulation of the Schaffer collaterals to CA1 hippocampus of pre-pubertal wild-type (left) but not pubertal wild-type (middle) hippocampus, an impairment reversed by δ knock-out (right). B, An active avoidance task where mice are trained to avoid an electrified sector (blue hatch marks) of a rotating disk. C, Representative paths of a wild-type and α4 knock-out mouse during avoidance learning. D, Nearly 10 x more α4 knock-out mice reached learning criterion compared to wild-type. (Shen et al., Science 2010; Shen et al., Brain Research 2016).
Inhibition and synaptic pruning during adolescence
The presence of the α4βδ GABAA receptor on dendritic spines of CA1 hippocampal pyramidal cells motivated my laboratory to investigate the role of this receptor in eliminating dendritic spines during adolescence. This process, known as "synaptic pruning" is believed to remove unnecessary synapses during adolescence to enable more relevant memories to form in adulthood. Our recent paper (Afroz, Parato et al., eLife 2016) shows that α4βδ GABAA receptors are the initial trigger for this process which leads to decreases in Kalirin-7, a spine protein which stabilized the actin cytoskeleton and is necessary for spine stability. In the α4 knock-out pruning is prevented and reversal learning (a form of cognitive flexibility) is impaired. Abnormal pruning has been reported in neuropsychiatric disorders such as autism and schizophrenia, where genetic abnormalities in α4 and δ expression are also found. Thus, our findings may suggest future therapeutic strategies for these disorders.
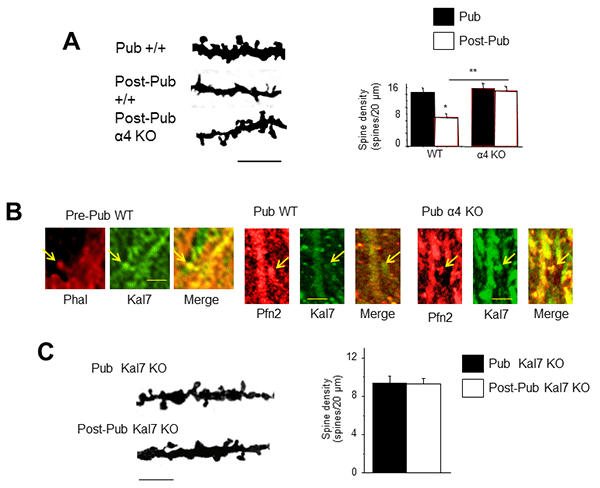
Figure 3. Pubertal α4βδ GABAA receptors trigger synaptic pruning of CA1 hippocampal pyramidal cells. A, Dendritic spine density decreases by half in adolescence in wild-type, but not α4 knock-out hippocampus. Left, Representative dendrites; right, Averaged data. B, The spine protein, kalirin-7 (Kal7) decreases at puberty in wild-type (middle) but not α4 knock-out (right) hippocampus compared to pre-pubertal (left). C, Synaptic pruning is not observed in the Kal7 knock-out hippocampus suggesting that dynamic changes in expression of this protein mediate pruning as a response to changes in α4βδ expression. Left, Representative dendrites; right, Averaged data. (Afroz, Parato et al., eLife 2016).
Inhibition and seizure activity during adolescence
The increase in α4βδ GABAA receptors at puberty also reduces the ability of high potassium to generate seizure-like activity in the CA1 hippocampus (Yang at el., Scientific Reports 2016) in studies performed in collaboration with Lisa Merlin. These findings may, at least in part, explain the dramatic 50% remission rate reported for childhood epilepsy during adolescence.
Neurosteroids and anxiety during adolescence
α4βδ GABAA receptors are the most sensitive targets for neurosteroids such as allopregnanolone (THP), a metabolite of progesterone. Before puberty and in the adult, this steroid, which is released in the brain in response to stress, enhances inhibition and reduces anxiety via limbic circuits which include the hippocampus. However, at puberty, when these α4βδ GABAA receptors are expressed in the CA1 hippocampus, allopregnanolone reduces their inhibitory effect, due to the direction of Cl- flux, because allopregnanolone creates inward rectification at the GABAA receptor-associated Cl- channel. This neurosteroid-induced reduction of inhibition increases neuronal excitability within the hippocampal circuit and behaviorally, results in increased anxiety as a result of stress only during the pubertal period (Shen et al., Nature Neuroscience 2007). These findings suggest that stress-induced anxiety is greater during adolescence which may underlie the mood swings and anxiety sometimes associated with this developmental period.
PersonnelHui Shen, M.D., Ph.D.
Research Associate Professor
Julie Parato
Graduate Student
Matthew Evrard
Graduate Student
Michael Tekin
Graduate Student
Ad hoc reviewer for the MDCN-P57 NIH study section
Ad hoc reviewer for various journals
Editor, Special issue of Brain Research: Adolescence as a Critical Period for Developmental Plasticity
- Smith, S. S., Gong, Q. H., Hsu, F. C., Markowitz, R. S., ffrench- Mullen, J. M. H., and Li, X. (1998). GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392, 926-929.
- Smith, S. S., and Gong, Q. H. (2005). Neurosteroid administration and withdrawal alter GABAA receptor kinetics in CA1 hippocampus of female rats. J. Physiol. 564, 421-436.
- Shen, H., Gong, Q.-H., Aoki, C., Yuan, M., Ruderman, Y., Dattilo, M., Williams, K., and Smith, S. S. (2007). Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nature Neurosci. 10, 469-477.
- Shen, H., Sabaliauskas, N., Sherpa, A., Fenton, A. A., Stelzer, A., Aoki,, C., Smith, S. S. (2010) A critical role for α4β2δ GABAA receptors in shaping learning deficits at puberty in mice. Science 327, 1515-1518.
- Shen, H., Mohammad, A., Ramroop, J., Smith, S. S. (2013). A stress steroid triggers anxiety via increased expression of α4β2δ GABAA receptors in methamphetamine dependence. Neuroscience 254, 452-475. Doi: 10.1016/j.neuroscience.2013.08.033.
- Gong, Q. H. and Smith, S. S. (2014). Characterization of neurosteroid effects on hyperpolarizing current at α4β2δ GABAA receptors. Psychopharmacology(Berl). 231, 3525-3535.
- Sabaliauskas, N., Shen, H., Molla, J., Gong, Q. H., Kuver, A., Aoki, C., and Smith, S. S. (2015). Neurosteroid effects at α4β2δ GABAA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 1621, 170-186. Special issue: "Brain and Memory: Old Arguments and New Perspectives" (G. Lynch, M. Baudry, eds.)
- Shen, H., Sabaliauskas, N., Yang, Y. Aoki,, C., Smith, S. S. (2016). Role of α4-containing GABAA receptors in limiting synaptic plasticity and spatial learning of female mice during the pubertal period. Brain Res. 1654 (Part B),116-122. Special issue "Adolescent Plasticity"
- Afroz, S., Parato, J., Shen, H., and Smith, S. S. (2016). Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAAreceptors on dendritic spines. eLife pii: e15106.
- Yang, L., Shen, H., Merlin, L. R., and Smith, S. S. (2016) Pubertal expression of α4β2δ GABAA receptors reduces seizure-like discharges in CA1 hippocampus. Sci. Rep. 6, 31928.