
Katherine L. Perkins, PhD
Associate Professor
Physiology and Pharmacology
GABA, which is released from interneurons, is the main inhibitory neurotransmitter in the brain. However, under certain circumstances, GABA depolarizes and excites the postsynaptic pyramidal cell and can actually trigger epileptiform activity. In addition, certain interneurons in the hippocampus are excited by exogenously applied GABA and by GABA released from other interneurons. We are studying these interneuron networks and the role they play in the epileptiform activity that occurs in the hippocampus and entorhinal cortex.
We have been using cell-attached and whole cell voltage-clamp techniques to record from interneurons and pyramidal cells during synchronous population activity in the hippocampus. Cell-attached techniques allow non-invasive recording of action potential currents and slow synaptic potentials from neurons in the brain slice (Perkins, 2006). In particular, cell-attached recording allows the polarity of GABA-mediated synaptic events (depolarizing or hyperpolarizing?) to be ascertained without affecting that polarity. These studies have identified a population of interneurons in hippocampal stratum lacunosum-moleculare (SLM) that receive a GABA-mediated depolarization that causes them to fire action potentials. These SLM interneurons may be responsible for the postsynaptic depolarizing GABA response that occurs in CA3 pyramidal cells because the action potentials in the SLM interneurons occur with the right timecourse, firing late in the population event (Fig. 1). Furthermore, application of the GABA-B antagonist CGP 55845 causes an increase in interneuron firing simultaneous with an enhancement of the depolarizing GABA response in the pyramidal cells (Fig. 1). This enhanced depolarizing GABA response that occurs in the presence of GABA-B antagonist is capable of triggering and sustaining epileptiform events (Kantrowitz et al., 2005).
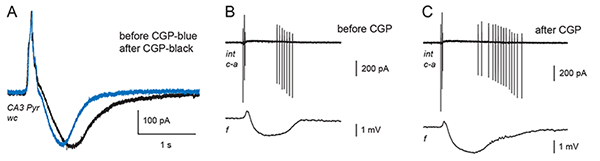
Figure 1. Addition of the GABA-B antagonist CGP 55845 lengthens the train of action potentials in the CA3 SLM interneuron and lengthens the depolarizing GABA response in the CA3 pyramidal cell.
A: Whole cell voltage-clamp recording (wc) of a spontaneous giant GABA-mediated postsynaptic current (GPSC) in a CA3 pyramidal cell with the GABA-B component already blocked from the inside with QX-314. The intracellular solution is high in bicarbonate (49 mM) in order to accentuate the depolarizing GABA response, which is seen here as an inward (downward-going) current. Addition of CGP 55845 (black trace) lengthens the depolarizing GABA response via an effect on the presynaptic interneurons. Holding potential -53 mV. (Adapted from Fig. 6B in Kantrowitz et al., 2005.)
B and C: Cell-attached recording (c-a) of action potential currents from a single interneuron residing in stratum lacunosum-moleculare (in a different brain slice from A). A simultaneous field potential (f) is recorded in distal SR of CA3. The interneuron fires in a distinctive pattern coincident with the spontaneous giant GABA-mediated postsynaptic potential (GPSP) seen in the field potential trace. C: Addition of CGP 55845 causes the interneuron to fire a longer burst of action potentials. The extracellular solution in A-C includes ionotropic glutamate receptor antagonists and 4-aminopyridine, which causes spontaneous, rhythmic GPSPs. (B and C: Yang and Perkins, in preparation).
In a second project, we have been examining the mechanism of epileptogenesis in entorhinal and perirhinal cortex in rodent brain slices (Salah and Perkins, 2011). Epileptogenesis is the process by which normal brain tissue is transformed into tissue that exhibits spontaneous epileptic activity. We have shown that the epileptogenesis in our model is NMDA-dependent and protein synthesis-independent. Furthermore, the epileptiform activity in this model is ictal-like, meaning that it looks like the activity that occurs in the intact brain during epileptic seizures (Fig. 2). Further investigating the mechanism of epileptogenesis in this in vitro model will hopefully shed light on the epileptogenesis that can occur in humans and offer possible interventions that may prevent epileptogenesis following brain injury.
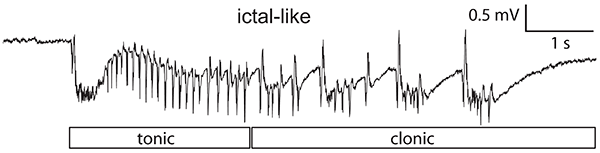
Figure 2. Application of 4-aminopyridine in solution containing 0.6 mM Mg2+ and 1.2 mM Ca2+ causes ictal-like activity in entorhinal cortex which remains after wash-out of the 4-aminopyridine. These individual ictal-like events have tonic- and clonic-like components and are over 4 seconds long, resembling the seizure activity that occurs in temporal lobe epilepsy patients.
A third project in the lab is the examination of the role of extracellular space volume in epileptiform activity. These experiments are being done in collaboration with Dr. Sabina Hrabetova's lab in the Department of Cell Biology. In our published work (Arranz et al. 2014), we showed that mutant mice deficient in the extracellular matrix component hyaluronan have epileptic seizures. They also have reduced extracellular space volume in the CA1 region of the hippocampus. Brain slices taken from these mice showed epileptiform activity originating in the CA1 region of the hippocampus. Increasing the extracellular space volume via application of hypertonic saline blocked the epileptiform activity. In our ongoing studies, we are examining the time course and mechanisms of the interplay between epileptiform activity and extracellular space volume in wildtype mice.
Service FunctionsReviewer for various scientific journals.
- Perkins, K. L., and Wong, R. K. S. (1996). Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J. Neurophysiol. 76, 3886-3894.
- Chen, Q. X., Perkins, K. L., Choi, D. W., and Wong, R. K. S. (1997). Secondary activation of a cation conductance is responsible for NMDA toxicity in acutely isolated hippocampal neurons. J. Neurosci. 17, 4032-4036.
- Perkins, K. L. and Wong, R. K. S. (1997). The depolarizing GABA response. Can. J. Physiol. Pharmacol.75, 516-519.
- Perkins, K. L. (1999). Cl- accumulation does not account for the depolarizing phase of the synaptic GABA response in hippocampal pyramidal cells. J. Neurophysiol. 82, 768-777.
- Perkins, K. L. (2002). GABA application to hippocampal CA3 or CA1 stratum lacunosum-moleculare excites an interneuron network. J. Neurophysiol. 87, 1404-1414.
- Kantrowitz, J. T., Francis, N. N., Salah, A., and Perkins, K. L. (2005). Synaptic depolarizing GABA response in adults is excitatory and proconvulsive when GABAB receptors are blocked. J. Neurophysiol. 93, 2656-2667.
- Perkins, K.L. (2006). Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Meth. 154, 1-18.
- Salah, A. and Perkins, K.L. (2008). Effects of subtype-selective group I mGluR antagonists on synchronous activity induced by 4-aminopyridine/CGP 55845 in adult guinea pig hippocampal slices. Neuropharmacology 55, 47-54.
- Salah, A. and Perkins, K.L. (2011). Persistent ictal-like activity in rat entorhinal/perirhinal cortex following washout of 4-aminopyridine. Epilepsy Research 94, 163 - 176.
- Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, and Yamaguchi Y. (2014). Hyaluronan Deficiency Due to Has3 Knock-out Causes Altered Neuronal Activity and Seizures via Reduction in Brain Extracellular Space. J. Neurosci. 34, 6164-6176.