
Ira S Kass, PhD
Professor
Anesthesiology
Physiology and Pharmacology
Aside from their sedative and unconscious inducing properties, anesthetics can also impact the brain resulting in deleterious, therapeutic or protecting effects. Our lab is interested in studying these divergent properties of anesthetics that have become focal points in the field of pediatric anesthesia, neuropsychiatric treatment and surgical interventions. Specifically, we are interested in the effects of neonatal exposure to anesthesia on later-life behavioral changes; anesthetics such as, ketamine's, role in the treatment of depression; and anesthetics as pre-conditioning agents for surgically induced cerebral ischemia.
Roles Of Anesthetics In The Developing Brain
Neonatal exposure to anesthetics is associated with social interaction and cognitive deficits later on in life (Lin et al. 2016). To understand the underlying mechanisms that result in long-term functional change, we are investigating the roles of microRNAs, neuronal circuitry and morphology during development. Our goal is to move toward therapeutic interventions to attenuate the risks associated with anesthetics given to young children.
Two Roles Of Ketamine: Anesthetic And Antidepressant
Ketamine is commonly used as an induction agent for unconsciousness in children and adults. However in recent years, ketamine has been discovered as a novel treatment drug for depression. Will early-life exposure to ketamine result in depressive-like behavior later on in life? This is a paradoxical effect that has been shown with traditional antidepressant drugs that are selective serotonin reuptake inhibitors (SSRIs), when applied during early brain development. Our lab has initiated behavioral paradigms, cellular, molecular and electrophysiological approaches to investigate the effects of ketamine on the developing brain and the mechanism of its efficacy as an antidepressant.
Anesthetics As A Preconditioning Agent For Cerebral Ischemia
We are also examining the protective effects of anesthetic preconditioning against hypoxic and ischemic brain damage in adult rats. Cerebral ischemia is a frequent consequence during cardiac and neurological surgery. In surgical procedures with a high risk of ischemia, it is important to choose an anesthetic that offers protection. A focus of our studies is to examine how anesthetics work on the cellular and molecular level to enhance recovery after an ischemic insult. We found that the volatile anesthetic sevoflurane improves recovery after hypoxia by activating the mTOR pathway and increasing the expression of protein kinase Mζ. Additionally, we found that the local anesthetic and antiarrhythmic agent lidocaine reduces neuronal damage after hypoxia, an effect that is associated with reduced neuronal cell loss in the CA1 region of the hippocampus and improved learning and memory 4 weeks after ischemia.
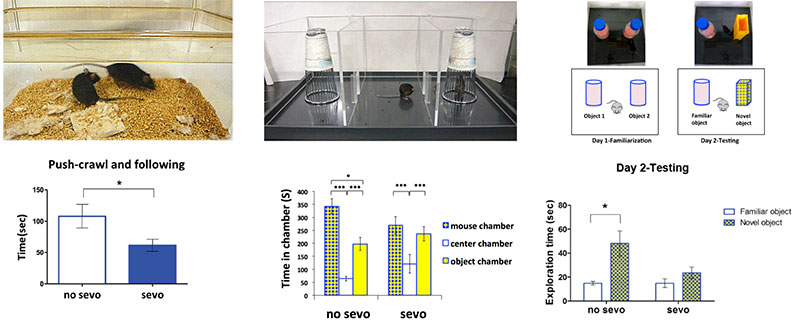
Postnatal day 7 (P7) sevoflurane treated mice showed significant impaired social interactions in adulthood as shown by reciprocal social interaction (Figure 1) and three chamber social interaction (Figure 2) when compared to the no sevo treated mice. While the no-sevo treated group spent significantly more time exploring the novel object, the P7 sevo treated group did not show an increased interest when examined during adult (Figure 3).
PersonnelDaisy Lin, Ph.D., Assistant Professor
Baiping Lei, M.D., Ph.D., Clinical Assistant Professor
Jinyang Liu, M.S., Research Scientist
Editorial Board: Journal of Neurosurgical Anesthesiology, Raven Press.
- Kass, I. S., and Lipton, P. (1982). Mechanisms involved in irreversible anoxic damage to the in vitro rat hippocampal slice. J Physiol. 332, 459-472.
- Kass, I. S., and Lipton, P. (1989). Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J. Physiol. 413, 1-11.
- Fried, E., Amorim, P., Chambers, G., Cottrell, J. E., and Kass, I.S. (1995). The importance of sodium for anoxic transmission damage in rat hippocampal slices: mechanisms of protection by lidocaine. J Physiol. 489, 557-565.
- Lei, B., Popp, S., Capuano-Waters, C., Cottrell, J. E., and Kass, I. S. (2004). Lidocaine attenuates apoptosis in the ischemic penumbra and reduces infarct size after transient focal cerebral ischemia in rats. Neuroscience 125, 691-701.
- Popp, S. S., Lei, B., Kelemen, E., Fenton, A. A., Cottrell, J. E., and Kass, I. S. (2011). Intravenous antiarrhythmic doses of lidocaine increase the survival rate of CA1 neurons and improve cognitive outcome after transient global cerebral ischemia in rats. Neuroscience 192, 537-549.
- Wang, J., Meng, F., Cottrell, J. E., Sacktor, T. C., and Kass, I. S. (2012). Metabotropic actions of the volatile anaesthetic sevoflurane increase protein kinase M synthesis and induce immediate preconditioning protection of rat hippocampal slices. J Physiol. 590, 4093-4107.
- Peng, Y., Zhang, W., Kass, I. S., and Han, R. (2016). Lidocaine reduces acute postoperative pain after supratentorial tumor Surgery in the PACU: A Secondary Finding From a Randomized, Controlled Trial. J. Neurosurg. Anesthesiol. 28, 309-315.
- Lin, D., Liu, J., Kramberg, L., Ruggiero, A., Cottrell, J., and Kass, I. S. (2016). Early-life single-episode sevoflurane exposure impairs social behavior and cognition later in life. Brain Behav. 6, e00514.
- Liu, J., Yang, L., Lin, D., Cottrell, J. E., and Kass, I. S. (2018). Sevoflurane blocks the induction of long-term potentiation when present during, but not when present only before, the high-frequency stimulation. Anesthesiology 128, 555-563.
- Lin, D., Liu, J., Hu, Z., Cottrell, J. E., and Kass, I. S. (2018). Neonatal anesthesia exposure impacts brain microRNAs and their associated neurodevelopmental processes. Sci. Rep. 8, 10656.