
Steven E. Fox, PhD
Professor
Physiology and Pharmacology
This laboratory uses electrophysiological and pharmacological methods to study the EEG theta rhythm in rats to understand how the associated brain state influences information processing by the hippocampus. Theta rhythm occurs during movement and is paced by cholinergic neurons that project from the medial septal nuclei to the hippocampus. The degeneration of these basal forebrain cholinergic neurons is probably the cause of the memory deficit of Alzheimer's patients.
The hippocampal theta rhythm is an ideal model system for studying the generation of rhythmic slow waves in the brain. The mechanisms of naturally occurring slow waves are poorly understood at the present time, yet this information is crucial for understanding the generation of pathological slow waves. Knowledge of the mechanisms of theta rhythm may also suggest hypotheses about its function. The role of the hippocampus in mnemonic processes, the cholinergic nature of a component of the theta rhythm and the presence of theta rhythm in primates suggest that part of the symptomatology of Alzheimer's disease may be related to loss of the theta rhythm. Recordings from hippocampal pyramidal cells in freely-moving rats have shown that they fire most rapidly when the rat is in a particular part of its environment, as if they were extracting "place" information from multimodal sensory cues. The regions where these units exhibit such high firing rate are termed "place fields". The walking-induced theta rhythm occurs naturally as the rat moves from place to place and it represents oscillations of the membrane potentials of these same pyramidal cells, so it may reflect this extraction process. Our work has indicated that a) the place field is enhanced during theta rhythm by increased firing inside the field and decreased firing outside the field, and b) the timing of the relationship between the firing of the cells and the waveform of the theta rhythm changes as the rat passes through the place field. This laboratory performs studies designed to contribute to our understanding of the synaptic mechanisms of these two effects. First, further quantitative descriptive studies on the two effects are necessary to characterize them more completely. Secondly, the synaptic mechanisms leading to these two effects are studied by administration of drugs that activate or interfere with specific neurotransmitter systems that are suspected sources of such changes. Initially, these studies center around three important transmitters that are central to hippocampal function: acetylcholine, GABA and glutamate. We study these systems by applying neurotransmitter specific drugs to the area surrounding our unit recording electrode and observing the effect on our recording. In this way the drug influences the activity of only a small subset of hippocampal neurons, keeping behavior constant so the incoming activity is controlled. We should be able to mimic components of the changes in behaviors where they do not ordinarily occur and block them in the behaviors where they ordinarily would occur. These studies will provide critical tests of the specific features of our hypothetical model for the role of the theta state in hippocampal function.
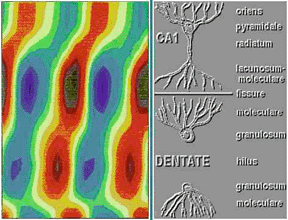
Figure 1. Above is a depth profile of the AC component of the hippocampal theta rhythm recorded from a rat during walking. The X axis is phase of the theta rhythm across two cycles. The Y axis is depth in the hippocampus through CA1 and both blades of the dentate gyrus. Voltage is coded as colors with warm colors representing positivity and cool colors representing negativity. The amplitude maximum occurs at the hippocampal fissure. Note that the phase shifts slowly through CA1, shifts again near the dorsal blade of the granule cell layer and then shifts back at the ventral blade.
PersonnelElena S. Brazhnik, Ph.D., Senior Research Scientist
-
Brankack, J., Stewart, M., and Fox, S. E. (1993). Current source density analysis of the hippocampal theta rhythm: Associated sustained potentials and candidate synaptic generators. Brain Res. 615, 310-327.
-
Brazhnik, E. S., Muller, R. U., and Fox, S. E. (2003). Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J. Neurosci. 23, 611-621.
-
Brazhnik, E. S. Borgnis, R., Muller, R. U., and Fox, S. E. (2004). The effects on place cells of local scopolamine dialysis are mimicked by a mixture of two specific muscarinic antagonists. J. Neurosci. 24, 9313-9323.
-
Barry, J. M., Rivard, B., Fox, S. E., Fenton, A. A., Sacktor, T. C., and Muller, R. U. (2012). Inhibition of protein kinase Mzeta disrupts the stable spatial discharge of hippocampal place cells in a familiar environment. J. Neurosci. 32, 13753-13762.
-
Kubie, J. L., and Fox, S. E. (2015). Do the spatial frequencies of grid cells mold the firing fields of place cells? Proc. Natl. Acad. Sci. USA 112, 3860-3861.
-
Fox, S. E. and Ranck, J. B. (2018). measurement of the topological dimension of hippocampal place cell activity. bioRxiv, https://doi.org/10.1101/442905.
-
Bolding, K. A., Ferbinteanu, J., Fox, S. E., and Muller, R. U. (2020). Place cell firing cannot support navigation without intact septal circuits. Hippocampus 30, 175-191.