
Diana Dow-Edwards, PhD
Professor Emeritus
Physiology and Pharmacology
Anatomy and Cell Biology
Our lab is currently inactive. However, we have studied the effects of addictive drugs on the functional ontogeny of the CNS, during both prenatal and adolescent development, with a special emphasis on male-female differences. These highly translational studies have contributed greatly to understanding brain-behavior relationships and how drug exposure during development alters the neural circuit underpinnings of behavior. Recently, our focus has been on marijuana. Following my Fulbright experience (2015-2016 Distinguished Visiting Research Chair in Brain Science, Child and Family Health and Wellness), I have been lecturing to lay and professional audiences throughout the US and Canada on the effects of marijuana on brain development.
Video: Marijuana and the Development of the Brain
Adolescent THC and the effects of early stress (thesis work of Lindsay Silva, PhD).
Adolescents who use marijuana are more likely to exhibit anxiety, depression, and other mood disorders, including psychotic-like symptoms. To examine the effects of early life experience on adolescent Δ-9-tetrahydrocannabinol (THC) exposure, we exposed adolescent male and female rats, either shipped from a supplier or born in our vivarium, to once daily injections of THC. Results suggest that males are more sensitive to the anxiolytic and antidepressant effects of THC than females and shipping increased THC responses primarily in males. THC increased cannabinoid receptor 1 (CB1R) mRNA expression and decreased CP55,940 stimulated [35S]GTPγS binding in the central amygdala only of females. Therefore, early stress enhances THC responses and THC alters CB1R expression and function in females only. In summary, THC and stress interact with the developing endocannabinoid system in a sex specific manner during the peri-pubertal period.
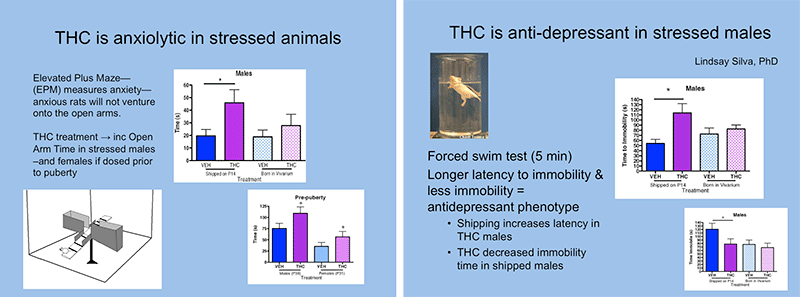
Figure 1. Effects of adolescent THC exposure on anxiety and depressive-like behavior in stressed and non-stressed rats. Shipping stress at P14 increased the response to chronic THC (3mg/kg) if the THC is administered during the prepubescent period.
Prenatal cocaine alters the rewarding effects of cocaine in adolescent rats.
Gestational exposure to cocaine now affects several million people including adolescents and young adults and may alter vulnerability to take and/or abuse illicit drugs. This study sought to answer the question "Does prenatal exposure to cocaine, in a dose-response fashion, alter the rewarding effects of cocaine using a conditioned place preference (CPP) procedure during adolescence in the rat, and are there sex differences and what is the effect of environment?"
Rats were dosed daily with cocaine at two doses prior to mating and throughout gestation. On postnatal day (PND) 23, pups were placed into isolation cages or enriched cages. On PND43-47, CPP was determined across a range of cocaine doses. Results show that prenatal cocaine increased sensitivity to the rewarding effects of cocaine in adolescent males, and being raised in an enriched environment further enhanced this effect. Rats exposed to the high dose resembled the controls.
These data support the unique sensitivity of males to the effects of gestational cocaine, that moderate prenatal cocaine doses produce greater effects on developing reward circuits than high doses and that housing condition interacts with prenatal treatment and sex such that enrichment increases cocaine CPP mostly in adolescent males prenatally exposed to moderate cocaine doses.
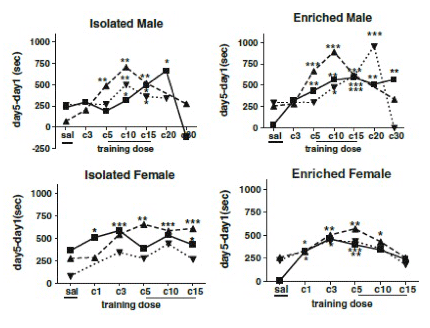
Figure 2. Conditioned place preference (Time on drug side on day 5-day 1 in seconds) in male and female rats at PND 49 for several training doses of cocaine (X-axis). Prenatal treatments are vehicle ■, cocaine 30mg/kg/day▲, cocaine 60 mg/kg/day▼. Overall analysis utilized mixed linear models comparing saline and C5, C10, and C15 doses (underlined) indicated that scores of all these training doses were significantly different from saline. Within prenatal treatments, sexes and housing conditions, scores of individual training doses were subjected to t tests (compared to zero) *** significantly different from 0 at p<0.001; ** significantly different from 0 at p<0.01; *significantly different from 0 at p<0.05; no * indicates group scores not different from 0 (no preference).
Single trial nicotine conditioned place preference in pre-adolescent male and female rats.
60% of tobacco smokers start before age 14. Females are typically more sensitive to nicotine than males yet few studies examine the effects of nicotine on reward systems in preadolescent female subjects. This study utilized single trial conditioned place preference (CPP) test in juvenile rats of both sexes and found that 0.05 mg/kg nicotine induced CPP in females while both sexes showed CPP following the higher dose. Nicotine also had substantial lasting behavioral effects at doses substantially lower than seen at older ages reported by others. These effects, which could potentially result from cigarette or e-cigarette smoking by 11-12 year old children, focus attention on the vulnerability of this age group to nicotine and the heightened sensitivity of females.
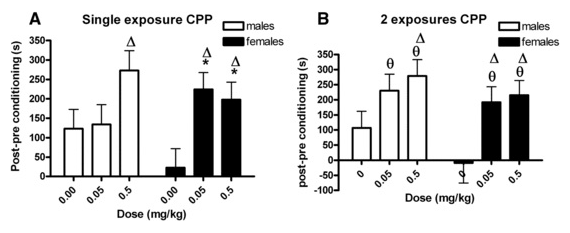
Figure 3. Time spent on drug side of conditioning chamber following conditioning minus time spent on drug side prior to conditioning (CPP) in pre-adolescent male and female rats following a single nicotine injection (A) or two nicotine pairings (B). * significantly different from saline; θ significantly different from saline when collapsed across sex. Δ significantly different from zero (0 = no preference) by Bonferroni-corrected t test. (mean ± sem) From: Edwards et al, 2014.
Service:Ad hoc member of multiple NIH study sections.
- DiNieri, J. A., Wang, X., Szutorisz, H., Spano, S. M., Kaur, J., Casaccia, P., Dow-Edwards, D., and Hurd, Y. L. (2011). Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 70, 763-769.
- Harte-Hargrove, L. C., and Dow-Edwards, D. L. (2012). Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav. Brain Res. 231, 48-59.
- Silva, L., Zhao, N., Popp, S., and Dow-Edwards, D. (2012). Prenatal tetrahydrocannabinol (THC) alters cognitive function and amphetamine response from weaning to adulthood in the rat. Neurotoxicol. Teratol. 34, 63-71.
- Edwards, A. W., Konz, N., Hirsch, Z., Weedon, J., and Dow-Edwards, D. L. (2014). Single trial nicotine conditioned place preference in pre-adolescent male and female rats. Pharmacol. Biochem. Behav. 125, 1-7.
- Dow-Edwards, D., Iijima, M., Stephenson, S., Jackson, A. and Weedon, J. (2014). The effects of prenatal cocaine, post-weaning housing and sex on conditioned place preference in adolescent rats. Psychopharmacology 231, 1543-1555.
- Silva, L., Harte-Hargrove, L., Izenwasser, S., Frank, A., Wade, D., and Dow-Edwards, D. (2015). Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci. Lett. 602, 89-94.
- Silverman, N. S., Popp, S., Vialou, V., Astafurov, K., Nestler, E. J., and Dow-Edwards, D. (2016). Effects of gaboxadol on the expression of cocaine sensitization in rats. Exp. Clin. Psychopharmacol. 24, 131-141.
- Silva, L., Black, R., Michaelides, M., Hurd, Y. L., and Dow-Edwards, D. (2016). Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol. Teratol. 58, 88-100.
- Dow-Edwards, D., Frank, A., Wade, D., Weedon, J., and Izenwasser, S. (2016). Sexually-dimorphic alterations in cannabinoid receptor density depend upon prenatal/early postnatal history. Neurotoxicol. Teratol. 58, 31-39.
- Dow-Edwards, D. and Silva, L. (2017). Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 1654(Pt B), 157-164.