
Matthew R. Pincus, PhD
Director, Mirimus Molecular Diagnostic Laboratories
Professor
Department of Pathology
Dr. Pincus is a board-certified and internationally recognized clinical pathologist. He received his MD and PhD degrees (with Dr. Robert P. Carty in Biochemistry) from SUNY Downstate Medical Center. He performed his residency in Pathology at Columbia College of Physicians and Surgeons in New York City, and was a postdoctoral fellow in Polymer Chemistry at the Weitzman Institute of Science in Israel and then at Cornell University in Chemical Physics (protein folding) with Professor Harold A. Scheraga.
Dr. Pincus was an Expert-Consultant in the Laboratory of Mathematical Biology at the National Cancer Institute. He was a professor of pathology at New York University Medical Center and was Chief of Pathology at Gouverneur Hospital and Director of Clinical Chemistry at Bellevue Hospital and then Professor of Pathology at SUNY Health Science Center at Syracuse. He became Professor of Pathology at SUNY Downstate Medical Center and Chairman of the Departments of Pathology and Laboratory Medicine at the New York Harbor VA Medical Center.
Dr. Pincus is currently Professor of Pathology at SUNY Downstate Medical Center and Director of Mirimus Molecular Diagnostic Testing Laboratories. He is the Co-Editor of the internationally renowned textbook in Clinical Pathology (Laboratory Medicine), Henry's Clinical Diagnosis and Management by Laboratory Methods. He has published over 300 peer-reviewed papers in basic science and clinical Medicine and has designed and tested a new anti-cancer peptide, PNC-27, that kills cancer cells by a novel mechanism but has no effect on normal cells which will soon be entering clinical trials based on this work. He is currently developing a method enabling identification of cancer cells at early stages of development.
Dr. Pincus has received numerous academic and teaching awards including Millenial GEM Lecturer (SUNY Downstate Alumni Association), the Jean R. Oliver Master Teaching Awards, Keynote Research Speaker Award (VA Medical Center) and Graduate Medical Education Award.
Education
Undergraduate: Bowdoin College
Advanced Degree: SUNY Downstate Health Sciences University
Medical School: SUNY Downstate Health Sciences University
Internship: Columbia College of Physicians & Surgeons
Residency: Columbia College of Physicians & Surgeons
Expert-Consultant: National Cancer Institute
Fellowship: Cornell University
Office
Department of Pathology
Room 4-122
SUNY Downstate Health Sciences University
Department of Pathology & Laboratory Medicine
Room 3-302
New York Harbor VA Medical Center,
800 Poly Place
Brooklyn, NY, 11203
Research Interests
Design of anti-cancer peptide agents, protein markers for cancer detection
Research Interest Summary
We have designed a group of anti-cancer peptides from the ras-p21 and p53 proteins that kill cancer cells but have no effect on normal cells.
Our inventions encompass 2 sets of peptides, one from the p53 anti-onogene protein and one from ras-p21 oncogenic protein. We have designed two sets of peptides from the ras-p21 and p53 proteins using computer-based molecular modeling of the three-dimensional structures of these proteins. We have synthesized three p53 peptides from its mdm-2-binding domain (i.e., residues 12-26, 17-26 and 12-20) each attached to a pentratin sequence that allows for its transport across the cell membrane. P53 12-26-penetratin, p53 17-26-penetratin and p53 12-20-penetratin are called PNC-27, 28 and 21, respectively. We first tested these peptides against two cell lines that we developed: a normal pancreatic acinar cell line, called BMRPA1, and its malignantly transformed counterpart cell line, called TUC-3, which we developed by transfecting the ras-oncogene into these BMRPA1 cells. When we incubated any of these peptides with TUC-3 cells, we found that all of them, but not a control peptide called PNC-29, killed all of the TUC-3 cells within 3 days but had no effect on the growth or viability of BMRPA1 cells. Significantly, we found that PNC-28 (p53 17-26-penetratin) had no effect on the ability of human stem cells from cord blood to differentiate into hematopoietic cell lines suggesting that this peptide would not suppress bone marrow. We have tested PNC-27 and 28 on 20 different human cancer cell lines and found that they are cytotoxic to all of them, inducing total cancer cell death in very short periods of time. For example, PNC-27 kills three different human breast cancer cell lines within 1 hour while having no effect on an untransformed human breast epithelial cell line. We have obtained evidence that the peptides work by interacting with two proteins that are present in the cell membranes of cancer but not normal cells. The peptides then induce membranolysis of the cancer cells. We have further tested our peptides against the highly malignant, metastatic TUC-3 pancreatic cancer cell line in nude mice and find that they eradicate the tumor within two weeks of drug delivery. This work has been published as the cover article in the September, 2006 issue of the International Journal of Cancer. It appears that this peptide would prove successful in treating a variety of human cancers.
We have further designed two peptides from the ras-p21 oncogenic protein using computer-based molecular modeling. These peptides correspond to amino acid residues 35-47 (PNC-7) and 96-110 (PNC-2). These two peptides selectively block oncogenic ras-p21 but not wild-type ras-p21 in a Xenopus (frog) oocyte model system. We have found that wild-type and oncogenic ras-p21 utilize different signal transduction pathways allowing for the oncogenic pathway to be selectively inhibited. We then proceeded to test these peptides on a ras-transformed pancreatic cancer cell line. In these experiments both peptides were linked to a penetratin peptide sequence that allows the peptide to be transferred across the cell membrane. This cell line, called TUC-3, was produced by transfecting the ras oncogene into a normal pancreatic acinar cell line, called BMRPA1. When we incubated either peptide with TUC-3 cells, the cells reverted back to the untransformed phenotype, i.e., became normal cells again. On the other hand, treatment of the normal acinar cells with either peptide had no effect either on their growth rates or on their viabilities. We have tested these two peptides on a number of different human cancer cell lines, i.e., fibrosarcoma, colon cancer, pancreatic cancer and astrocytoma, and found that both peptides either induce reversion to the untransformed phenotype or induce cancer cell death.
Funded by: VA and the American College of Surgical Oncology
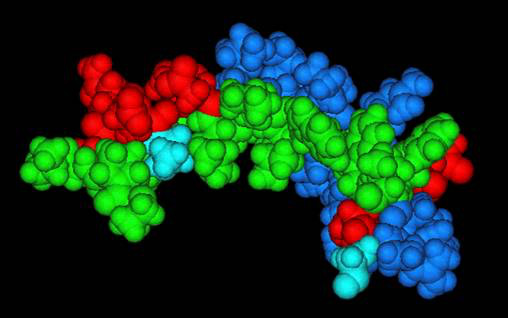
Space-filling representation of the three-dimensional structure of the anti-cancer peptide, PNC-27, showing the amphipathic nature of the molecule. The green domains show the hydrophobic face of the peptide while the red and blue show the positions of polar negativley and positive charged amino acid residues, respectively on the opposite face of the molecule. The positively and negatively charged residues are seen further to be separated from one another.
- Pincus MR, Silberstein M, Zohar N, Sarafraz-Yazdi E, Bowne WB (2024) Poptosis or Peptide-Induced Transmembrane Pore Formation: A NovelWay to Kill Cancer Cells without Affecting Normal Cells Biomedicines 12, 1144. https://doi.org/10.3390/biomedicines12061144.
- Krzesaj P, Adler V, Feinman RD, Miller A, Silberstein M, Yazdi E, Pincus MR (2024) Anti-Cancer Peptide PNC-27 Kills Cancer Cells by Unique Interactions with Plasma Membrane-Bound hdm-2 and with Mitochondrial Membranes Causing Mitochondrial Disruption. Ann Clin Lab Sci 54 (2), 137-147.
- Cheng HT, Premsrirut, P, Tennyson L, Mo P, Wang XH, Silberstein M, Pincus MR (2024) Unexpected Factors That Influence Viral Detection and Viral Load Using Real-Time PCR. Med Res Arch 12(5) https://doi.org/10.18103/mra.v12i5.5285.
- Pincus MR, Bowne WB and Sarafraz-Yazdi, E (2023) Poptosis: A Novel Mechanism for the Selective Killing of Cancer Cells Clin Oncol 7 (6), 1-13 , ISSN: 2640-1037.
- Miller AI, Diaz D, Lin B, Krzesaj PK, Ustoyev S, Shim A, Fine EJ, Sarafraz-Yazdi E, Pincus MR, Feinman RD (2023), Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents. Biomedicines 11, 2515. https://doi.org/10.3390biomedicines11092515.
- Seydfakan S, Atia H, Lin B and Pincus MR (2023) Age-Dependent Occurrence of Prostate Cancer in an African-American Patient Population of Predominantly West Indian Origins Med Res Arch (European Society of Medicine) Online 11 (8) https://doi.org/10.18103/mra.v11i8.4226.
- Christian M, Minkowitz J, Gabutan E, Bluth M, Steimetz E, Coca-Guzman J and Pincus MR (2023) Stability of Thyroid Function Test Analytes Whose Serum Levels Are Determined by Immunoassay Med Res Arch (European Society of Medicine) Online 11 (2) https://doi.org/10.18103/mra.v11i2.3606.
- Pincus MR, Lin B, Patel P, Gabutan E, Zohar N and Bowne W (2023) Peptides That Block RAS-p21 Protein-Induced Cell Transformation Biomedicines 11, 471-496, https://doi.org/10.3390/biomedicines11020471.
- Momani-Boroujeni A,Laskar D, Daich J, Seydafkan S and Pincus MR Combined Use of Troponin and CK-MB in the Prediction of Acute Myocardial Infarction in Patients with Acute Chest Pain EC Pharmacology and Toxicology 10 (6) May, 2022.
- Sarafraz-Yazdi E, Mumin S, Cheung D, Fridman D, Lin B,Wong L, Rosal R, Rudolph R, Frenkel M, Thadi A, Morano WF, Bowne WB, Pincus MR and Michl JP (2022) PNC-27, a Chimeric p53-Penetratin Peptide Binds to HDM-2 in a p53 Peptide-like Structure, Induces Selective Membrane-Pore Formation and Leads to Cancer Cell Lysis. Biomedicines10, 945; https://doi.org/10.3390/biomedicines10050945.
- Laskar DB, Minkowitz J, Boroujeni AM, Coca-Guzman J, Gabutan E, Bluth M, Michl JP, Zuretti A and Pincus MR (2022) Evaluation of the Efficacy of the Serodiagnostic Markers, CK-MB and Troponin, in the Acute Diagnosis of Myocardial Infarction. EC Pharmacology and Toxicology10(4) April, 2022.
- Miller A, Lin B, Pincus MR, Fine E and Feinman RD (2021) Selective Enhancement of Chemotherapeutic Agent-Induced Tumor Cell Killing by Acetoacetate and 3-Hydroxybutyrate EC Pharmacology and Toxicology 9, 29-34.
- Miller A, Lin B, Pincus MR, Fine E and Feinman RD (2021) Inhibition of cancer cells in culture. The effects of treatment with ketone bodies and/or rapamycin treatment bioRxiv https://doi.org/10.1101/2021.06.27.450024, 1-18.
- Seydafkan S, Minkowitz J, Li G, Cabenero M, Wang Z, Wang H, Alexis H, Eid I and Pincus MR (2021) Stability of Glucose Levels in Serum and Plasma Ann Clin Lab Sci 51, 589-591.
- Thadi A Morano WF, Khalili M, Babcock BD, Shaikh MF, Foster DS, Piazza Y, Gleeson EM, Goldstein E,Steele L, Campbell PM, Lin B, Pincus MR and Bowne WB (2021) Molecular Targeting of H/MDM-2 Oncoprotein in Human Colon Cancer Cells and Stem-like Colonic Epithelial-derived Progenitor Cells. Anticancer Res 41, 27-42.
- Seydafkan S, Minkowitz J, Li G, Cabenero M, Wang Z, Wang H, Alexis H, Eid I, Pincus MR. Stability of Glucose Levels in Serum and Plasma Ann Clin Lab Sci. 2021 Jul;51(4):580-583. PMID: 34452900
- Michl, J., Scharf, B., Schmidt, A., Hannan, R., von Gizycki, H., Friedman, F.K., Brandt-Rauf, P.W., Fine, R.L. and Pincus, M.R. (2006) PNC-28, a p53 Peptide that Is Cytotoxic To Cancer Cells, Blocks Pancreatic Cancer Cell Growth in vivo. Int. J. Cancer, 119, 1577-1585.
- Qu, Y., Adler, V., Izatova, L., Pestka, S., Bowne, W., Michl, J., Boutjdir, M., Friedman, F.K. and Pincus, M.R. (2007) The dual-specificity kinases, TOPK and DYRK1A, are critical for oocyte maturation induced by wild-type-but not by oncogenic- ras-p21 protein. Frontiers in Bioscience 12, 5089-5097.
- Pincus, M.R., Michl, J, Bowne, W. and Zenilman, M. (2007) Anti-Cancer Peptides from the ras-p21 and p53 Proteins. Research Advances in Cancer. R.M. Mohan, Ed. Global Research Network Publishers, Kerala, India, 65-90.
- Bowne, W., Michl, J., Bluth, M.H., Zenilman, M. and Pincus, M.R. (2007) Novel Peptides from the RAS-p21 and p53 Proteins for the Treatment of Cancer. Cancer Ther. 5, 331-346.
- Monaco, R., Rosal, R., Dolan, M.A., Pincus, M.R. and Brandt-Rauf, P.W. (2008) Conformational Effects of a Common Codon 399 Polymorphism on the BRCT1Domain of the XRCC-1 Protein. Protein J., in press.
- Adler, V., Bowne, W., Kamran, I., Michl, J., Friedman, F.K., Chin, E., Zenilman, M. and Pincus, M.R. (2007) Two Peptides Derived from ras-p21 Induce Either Phenotypic Reversion or Tumor Cell Necrosis of ras-Transformed Human Cancer Cells. Cancer Chemother Pharmacol. 62, 491-498.
- Adler, V., Bowne, W., Michl, J., Ikram, K., Pestka, S., Izatova, L., Zenilman, M., Friedman, F.K. and Pincus, M.R. (2007) Site-Specific Phosphorylation of raf in ras-Transformed Cells Mediated by jun-N-Terminal Kinase. Ann Clin Lab Med 38, 47-56
- HooKim, K., deRoux, S., Igbokwe, A., Stanek, A., Koo, J., Hsu, J., Pincus, M.R. and Bluth, M. H. (2007) IgG Anti-Cardiomyocyte Antibodies in Giant Cell Myocarditis. Ann clin Lab Med 38, 83-87.
- Cai, C.Q., Peng, Y., Buckley, M.T., Wei, J., Chen, F., Liebes, L., Gerald, W.L., Pincus, M.R., Osman, I. and Lee, P. (2007) Epidermal Growth Factor Receptor Activation in Prostate Cancer by Three Novel Missense Mutations. Onocogene 27, 3201-3210.
