
Weijun Jin, MD
Assistant Professor
Disorders of lipid metabolism are responsible for several important established risk factors for cardiovascular disease, which continues to be the leading cause of morbidity and mortality in the United States. Despite of decades of research, many aspects of lipid metabolism are incompletely understood.
The PC family is composed of the PCs furin, PC1/3, PC2, PC4, PACE4, PC5/6 isoform A and B, SKI-1/S1P, PC7 and NARC-1/PCSK9. PCs were suspected to be involved in lipid metabolism, but their importance has not been fully appreciated until the recent discoveries that mutations in PCSK9 are associated with autosomal dominant hypercholesterolemia in patients. Overexpression of PCSK9 resulted in a dramatic increase of low density lipoprotein cholesterol in mice (Figure 1), through both LDLR dependent and LDLR independent pathways. SKI-1, an ER-localized PC, cleaves membrane-bound sterol regulatory element binding proteins and releases the active subunit of SREBPs to maintain cellular cholesterol homeostasis
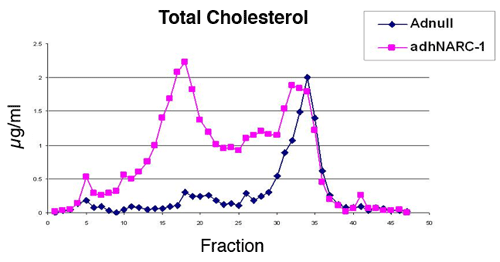
Figure 1
However, other classic members of the PC family regulate HDL metabolism through cleavage of substrates endothelial lipase and ANG PTL3 in vivo. Endothelial lipase is a lipolytic enzyme that hydrolyzes HDL phospholipids and here we showed that cleavage of endothelial lipase by classic PCs inactivates EL and results in an increase in HDL cholesterol levels in vivo. We also reported that ANG PTL3 is a natural inhibitor of endothelial lipase and that cleavage of ANG PTL3 by classic PCs converts a pro-form to a mature active form that is capable of inhibition of EL. Thus through both direct cleavage of EL as well as through activation of an endogenous inhibitor of EL, the classic PCs resulted in reduction in EL activity and increase in plasma HDL cholesterol levels. We demonstrated that EL is required for these effects of the classic PCs in that no effects on HDL are seen in EL deficient mice. Thus we establish that the classic PCs have a direct effect on regulating plasma HDL cholesterol levels much as the non-classic PCs have an effect in regulating LDL cholesterol levels (Figure 2).
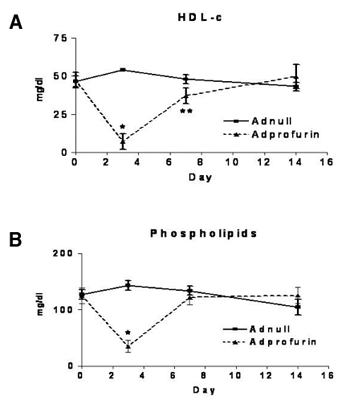
Figure 2
- Jin, W., Broedl, U.C., Monajemi, H., Glick, J.M., Rader, D.J.:Lipase H, a new member of triglyceride lipase family, is secreted in intestine. Genomics 80:268-272, 2002.
- Jin, W., Marchadier, D., Rader, D.J.: Lipases and HDL Metabolism. Trends in Endocrinology & Metabolism 13:174-178, 2002.
- Fuki, I.V., Blanchard, N., Jin, W., Marchadier, D.H., Millar, J.S., Glick, J.M., Rader, D.J.: Endogenously produced endothelial lipase enhances binding and cellular processing of plasma lipoproteins via HSPG-mediated pathway. Journal of Biological Chemistry 278:34331-34338, 2003.
- Jin, W., Millar, J.S., Broedl, U., Glick, J.M., Rader, D.J.: Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. Journal of Clinical Investigation 111:357-362, 2003.
- Jin, W., Sun, G.S., Marchadier, D., Octtaviani, E., Glick, J.M., Rader, D.J.: Endothelial cells secrete triglyceride lipase and phospholipase activities due to endothelial lipase in response to inflammatory cytokines. Circulation Research 92:644-50, 2003.
- Broedl, U.C., Maugeais, C., Millar, J.S., Jin, W., Moore, R.E., Fuki, I.V., Marchadier, D., Glick, J.M., Rader, D.J.: Endothelial Lipase Promotes the Catabolism of ApoB-Containing Lipoproteins. Circulation Research 94:1554-1561, 2004.
- Benjannet, S., Rhainds, D., Essalmani, R., Mayne, J., Wickham, L., Jin, W., Asselin, M.C., Hamelin, J., Varret, M., Allard, D., Trillard, M., Abifadel, M., Tebon, A., Attie, A.D., Rader, D.J., Boileau, C., Brissette, L., Chretien, M., Prat, A., Seidah, N.G.: NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the LDLR and LDL-cholesterol. Journal of Biological Chemistry 279:48865-75, 2004
- Kratky, D., Zimmermann, R., Wagner, E.M., Strauss, J.G., Jin, W., Kostner, G.M., Haemmerle, G., Rader, D.J., Zechner, R.: Endothelial lipase provides an alternative pathway for FFA uptake in lipoprotein lipase-deficient mouse adipose tissue. Journal of Clinical Investigation 115:161-7, 2005.
- Jin, W., Fuki, I.V., Seidah, N.G., Benjannet, S., Glick, J.M., Rader, D.J.: Proprotein convertases are responsible for proteolysis and inactivation of endothelial lipase. Journal of Biological Chemistry 280:36551-36559, 2005.
- Wang, X., Jin, W., Rader, D.J.: Upregulation of macrophage endothelial lipase by toll-like receptors 4 and 3 modulates macrophage interleukin-10 and -12 production. Circ Res. 2007 100:1008-1015, 2007.
- Jin, W., Wang, X., Millar, J.S., Quertermous, T., Rothblat, G.H.Glick, J.M., Rader, D.J.: Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6:129-136, 2007.
- Zaid, A., Roubtsova, A., Essalmani, R., Marcinkiewicz, J., Chamberland, A., Hamelin, J., Tremblay, M., Jacques, H., Jin, W., Davignon, J., Seidah, N.G., Prat, A.: Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 48:646-654, 2008.