
Mohamed Boutjdir, PhD
Professor, SUNY Downstate
Associate Chief of Staff for Research & Development,
New York Harbor Health Care System (VAMC)
The research interest of Dr. Boutjdir’s team is in cardiac channelopathies, Long QT Syndrome, sudden cardiac death and related health disparities. Cardiac arrhythmias confer a considerable burden of morbidity and mortality in industrialized countries. Although coronary artery disease and heart failure are the prevalent causes of cardiac arrest, in 5–15% of patients, structural abnormalities at autopsy are absent. In a proportion of these patients, mutations in genes encoding cardiac ion channels are documented (inherited channelopathies), but, to date, the molecular autopsy is negative in nearly 70% of patients. Emerging evidence indicates that autoimmunity and inflammation is involved in the pathogenesis of cardiac arrhythmias. In particular, several arrhythmogenic autoantibodies and cytokines targeting specific calcium, potassium, or sodium channels in the heart have been identified. Experimental and clinical studies demonstrate that these autoantibodies and cytokines for example can promote conduction disturbances and life-threatening tachyarrhythmias by inducing substantial electrophysiological changes. In 2017, we coined the term ‘autoimmune/inflammatory cardiac channelopathies’ to define this novel pathogenic mechanism of cardiac arrhythmias, which could be more frequent and clinically relevant than previously appreciated. Indeed, pathogenic autoantibodies against ion channels are detectable not only in patients with manifest autoimmune disease, but also in apparently healthy individuals, which suggests a causal role in some cases of unexplained arrhythmias and cardiac arrest. Considering this possibility and performing specific testing in patients with ‘idiopathic’ rhythm disturbances could create novel treatment opportunities.
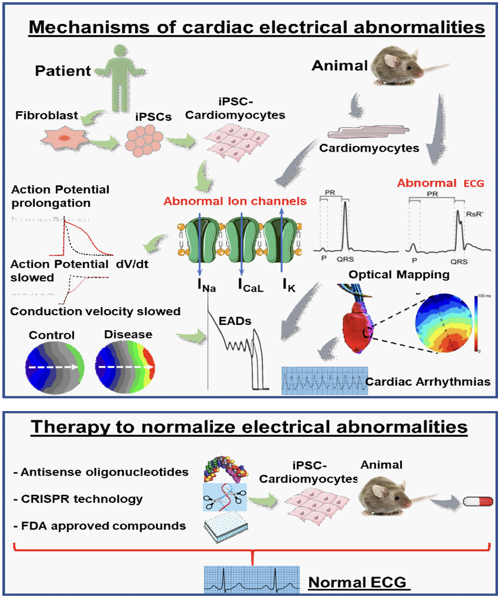
Expertise and Hands-On Technologies Used
Our approach to cardiac disease is to start from a clinical pathology/disease at the patient level and apply molecular, cellular, ex-vivo and in-vivo multidisciplinary approaches to identify the functional and biochemical pathways involved and then devise novel therapeutic approaches to treat the disease. The technologies used include state-of-the-art dual optical mapping systems, the patch-clamp technique, molecular and cellular biology as well as imaging technologies applied to human induced pluripotent stem cell derived cardiac microcytes (hiPSC-CMs) directly for patients, expression systems, cell lines and native cardiac myocytes.
- Lazzerini PE, Laghi-Pasini F, Boutjdir M*, Capecchi PL. Inflammatory cytokines and cardiac arrhythmias: the lesson from COVID-19. Nat Rev Immunol. 2022 Mar 28:1-3. doi: 10.1038/s41577-022-00714-3. (*) contributed equally.
- Lazzerini PE, Capecchi PL, Laghi-Pasini F, Boutjdir M. Autoimmune cardiac channelopathies: the heart of the matter. Nat Rev Cardiol. 2017 Sep;14(9):566.
- Lazzerini PE, Laghi-Pasini F, Boutjdir M*, Capecchi PL*. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019 Jan;19(1):63-64. (*) contributed equally
- Chahine M, Fontaine JM, Boutjdir M. Racial Disparities in Ion Channelopathies and Inherited Cardiovascular Diseases Associated With Sudden Cardiac Death.J Am Heart Assoc. 2022 Mar 15;11(6):e023446. doi: 10.1161/JAHA.121.023446. Epub 2022 Mar 4.
- Cardozo T, Cardozo L, Boutjdir M. Autoantibody:Autoantigen Competitor Decoys: Application to Cardiac Phenotypes. Front Immunol. 2022 Jan 28;13:812649. doi: 10.3389/fimmu.2022.812649. eCollection 2022.
- Poulin H, Mercier A, Djemai M, Pouliot V, Deschenes I, Boutjdir M, Puymirat J, Chahine M.
- iPSC-derived cardiomyocytes from patients with myotonic dystrophy type 1 have abnormal ion channel functions and slower conduction velocities. Sci Rep. 2021 Jan 28;11(1):2500.
- Lazzerini PE, Cevenini G, Qu YS, Fabris F, El-Sherif N, Acampa M, Cartocci A, Laghi-Pasini F, Capecchi PL, Boutjdir M*, Lazaro D*. Risk of QTc Interval Prolongation Associated with Circulating Anti-Ro/SSA Antibodies Among US Veterans: An Observational Cohort Study. J Am Heart Assoc. 2021 Feb 16;10(4):e018735.(*) contributed equally.
- Himel HD, Cupelli M, Boutjdir M, El-Sherif N. Voltage/Calcium Uncoupling Underlies Sustained Torsade de Pointes Ventricular Tachyarrhythmia in an Experimental Model of Long QT Syndrome. Front Physiol. 2021 Jan 28;12:617847.
- Lazzerini PE, Boutjdir M*, Capecchi PL*. COVID-19, Arrhythmic Risk, and Inflammation: Mind the Gap! Circulation. 2020 Jul 7;142(1):7-9. (*) contributed equally.
- Boutjdir M, Lazzerini PE. A Novel Peptide/Antibody-Based Antiarrhythmic Approach to Long QT Syndrome and Beyond. J Am Coll Cardiol. 2020 May 5;75(17):2153-2155.
Key words: Cardiac Electrophysiology, ion channels, long QT syndrome, Suden cardiac death, optical mapping, patch clamp, myotonic dystrophy, heart failure, autoimmune congenital heart block, channelopathies