
Shennan Aibel Weiss, MD, PhD
Assistant Professor
Physiology and Pharmacology
Neurology
Improving epilepsy surgery outcomes with fast ripple neurophysiological biomarkers
I am a clinical epileptologist and physician-scientist. One third of patients with focal epilepsy continue to have seizures despite optimal medical management. These patients are candidates for epilepsy surgery. The goal of my research is to improve the clinical outcomes of patients who undergo epilepsy surgery, so they achieve seizure freedom. To meet this goal, my lab studies the neural circuit mechanisms responsible for generating seizures and seizure-like activity. We have identified an integral role for fast ripples, which are brief bursts of energy (200-600 Hz) in the brain’s potential. A large clinical study (2023-2028) will assess if fast ripples can be used to plan more effective epilepsy surgeries.
High-frequency oscillations
A biomarker of a disease can be used for diagnosis. For example, the hemoglobin A1C level is a biomarker for the diagnosis of diabetes. Besides assisting diagnosis, an accurate biomarker should be intrinsically involved in the disease process and correlate with the disease severity. High-frequency oscillations (HFOs) are brief bursts of spectral energy found in brain recordings. One type of HFO known as a ripple (80-200 Hz) has been shown to be essential for memory consolidation in the hippocampus. However, in the context of epilepsy, fast ripples (200-600 Hz) are first seen following injuries and are generated by clusters of pathologically interconnected neurons. The generation of fast ripples predicts whether an injury will result in epilepsy. Also, in patients undergoing evaluation for epilepsy surgery with invasive intracranial electrodes the location of fast ripple generating tissue relative to the location of the operation can predict the success of surgery. We are interested in investigating fast ripples, for several reasons: 1) Fast ripples may be involved in triggering seizures and other epileptic activity; 2) Fast ripples propagate and form networks that may be linked to the severity of disease; 3) Characterizing fast ripple networks in patients undergoing evaluation for surgery may result in more successful operations.
Seizure initiation and spread
Seizures are known to be caused by abnormal electrical impulses in the brain. However, it is unclear if human seizures are caused by abnormal hyper-synchronization, or by heterogenous changes in firing? It is also unclear if the role of synaptic inhibition during a human seizure is to restrain the spread of the seizure, or if paradoxically abnormal synaptic inhibition can promote a seizure? My past work is consistent with the notion that, during a seizure, neurons are recruited in to two distinct territories: 1) a synchronized ictal core; and 2) a surrounding penumbra region where neuronal firing is heterogenous. The lab has also found that HFOs, and particularly fast ripples can also incrementally grow in power and frequency prior to seizure onset suggesting that some types of seizures maybe triggered when pathologically interconnected neurons synchronize and coalesce. Another important finding of the lab is that, in accord with electrophysiologic and optogenetic animal studies, in human recordings, at seizure spread, inhibitory interneurons increased their firing rate prior to the excitatory neurons. This work indicates that the neuronal underpinnings of seizures are likely diverse and may depend on the seizure morphology, the location of seizure onset, and the location of the analysis.
Pathological Phase-Amplitude Coupling
Phase amplitude coupling (PAC) occurs when the phase of a slower frequency modulates the amplitude of a faster frequency. PAC has been shown in human and animal experiments to be integral to cognition and memory. The lab has identified pathological PAC in patients with epilepsy when HFOs occur locked to consistent phases of ictal discharges. This form of PAC can be used to better identify epileptogenic regions. The lab also studies PAC during sleep in patients with epilepsy to distinguish HFOs produced by healthy brain tissue from pathologic HFOs based on coupling to the slow oscillation. This work may help to better discern the neural circuit mechanisms responsible for the pathological HFO activity, particular with respect to synaptic inhibition and a possible role of depolarizing inhibitory potentials. We have also begun to study pathological PAC during sleep in the framework of epileptic networks.
Figures
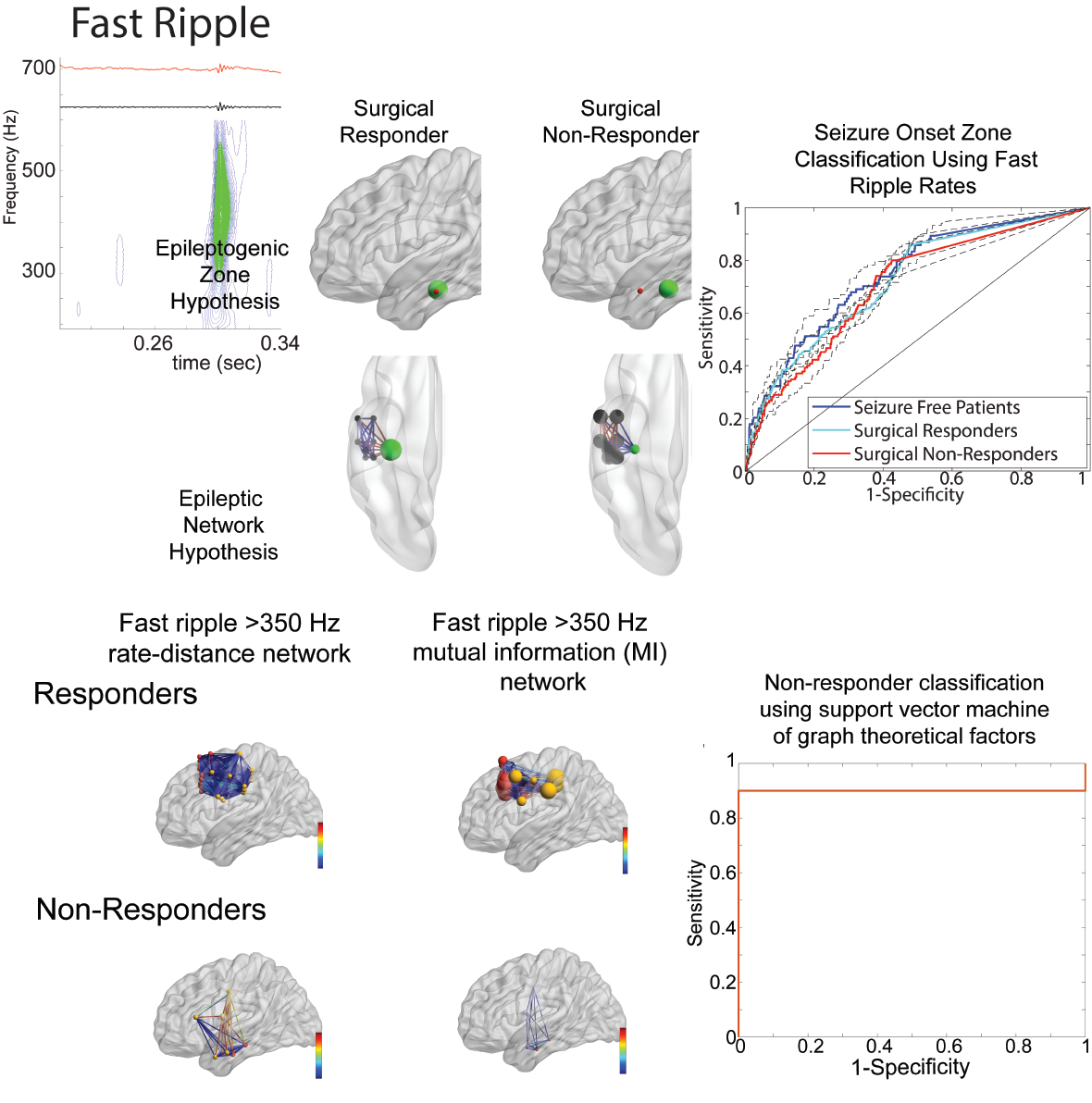
Figure 1. Graphical abstract showing that, as compared with the epileptogenic zone hypothesis (top), spatial and temporal correlational fast ripple networks (bottom) can better distinguish patients who improve after epilepsy surgery.
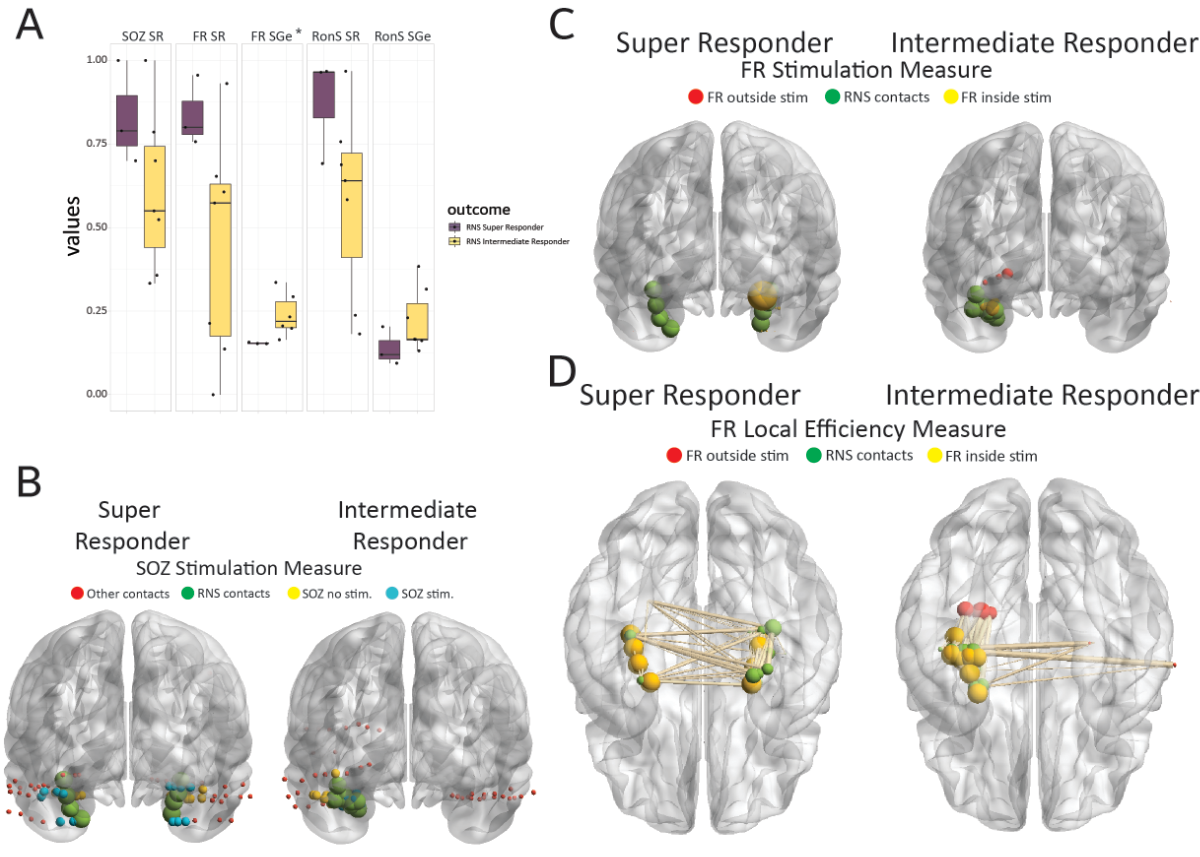
Figure 2. Stimulation targets fast ripple (FR)–generating networks, which are highly active and desynchronous, in super responders to the responsive neurostimulator system (RNS). (A) Box and scatter plots of the seizure-onset zone stimulation ratio (SOZ SR), FR stimulation ratio (FR SR), FR-stimulated global efficiency (FR SGe), ripple on spike (RonS) SR, and RonS SGe stratified by RNS seizure outcome of super responder and intermediate responder (*, p < .05). (B) Illustrative brain graph of the SEEG and RNS implants in a super responder (left) and intermediate responder (right). The RNS contacts are green, the stimulated (stim) SOZ contacts are cyan, the non-stimulated SOZ contacts are yellow, other contacts are red. (C) Illustrative brain graph as in B but only SEEG contacts generating FR are included. FR-generating contacts (i.e., nodes) that are stimulated are yellow, unstimulated FR-generating contacts are red. The size of the red and yellow nodes is proportional to the FR rate of that node. (D) Illustration of the FR mutual information network used to calculate the FR SGe. The nodes colored yellow are stimulated, and the nodes colored red are unstimulated. The size of the red and yellow nodes correspond to the local efficiency of the nodes. The edges are weighted by the FR mutual information value. Note that in C and in D the intermediate responder has more unstimulated FR nodes, and stimulated nodes with a high local efficiency and higher mutual information edges as compared to the super responder, respectively.
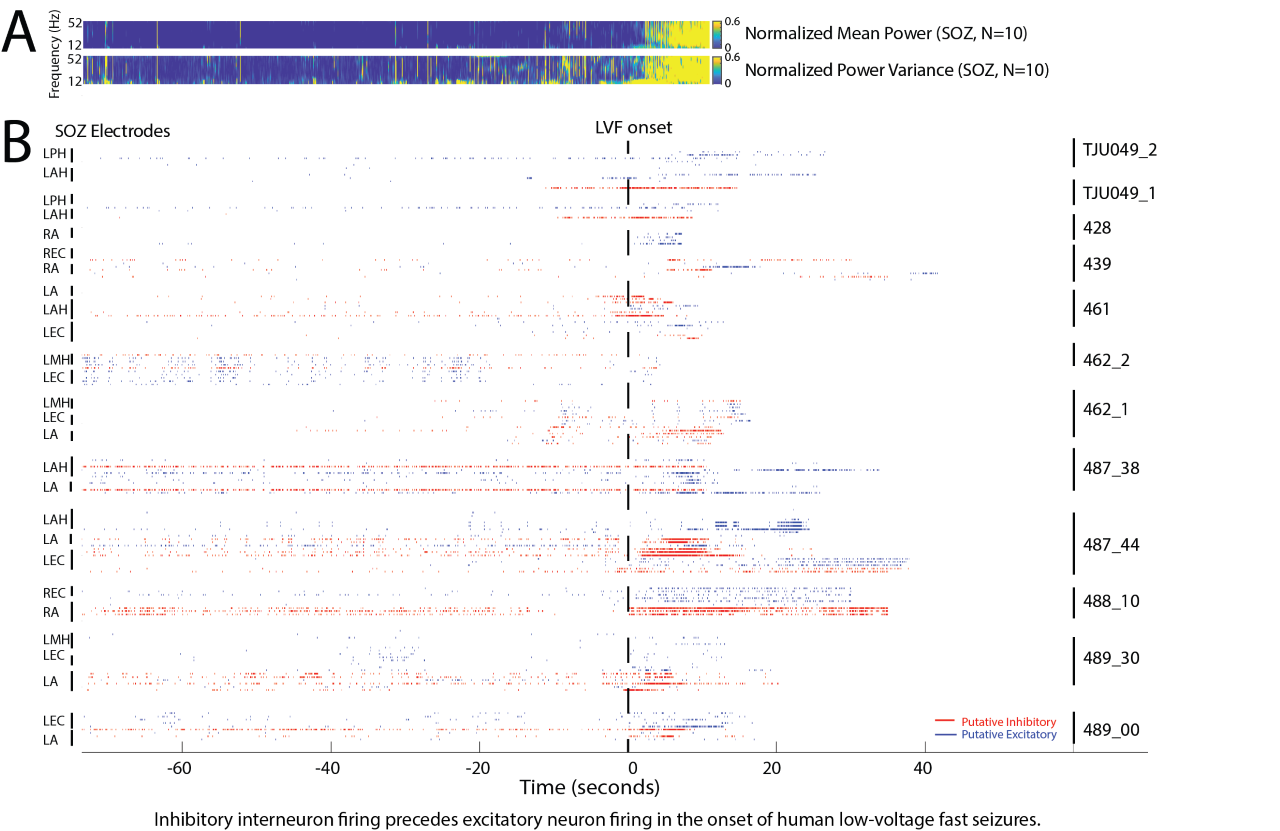
Figure 3. Raster plot of putative inhibitory (red) and excitatory (blue) neuronal action potential firing, isolated from microelectrodes implanted in patients undergoing pre-surgical evaluation, during spontaneous low-voltage fast onset focal seizures. Note that prior to, and during, the seizures the inhibitory neurons are active before the excitatory neurons.
- Weiss, S. A., Banks, G. P., McKhann, G. M., Goodman, R. R., Emerson, R. G., Trevelyan, A.J ., and Schevon, C. A. (2013). Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain 136, 3796–3808.
- Weiss, S. A., Lemesiou, A., Connors, R., Banks, G. P., McKhann, G. M., Goodman, R. R., Zhao, B., Filippi, C. G., Nowell, M., Rodionov, R., Diehl, B., McEvoy, A. W., Walker, M. C., Trevelyan, A. J., Bateman, L. .M, Emerson, R. G., and Schevon, C. A. (2015). Seizure localization using ictal phase-locked high gamma. Neurology 84, 2320–2328.
- Weiss, S.A., Alvarado‐Rojas, C., Bragin, A., Behnke, E., Fields, T., Fried, I., Engel, J., and Staba, R. (2016). Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia 57, 111–121.
- Song, I., Orosz, I., Chervoneva, I., Waldman, Z. J., Fried, I., Wu, C., Sharan, A., Salamon, N., Gorniak, R., Dewar, S., Bragin, A., Engel, J., Sperling, M. R., Staba, R., and Weiss, S. A. (2017). Bimodal coupling of ripples and slower oscillations during sleep in patients with focal epilepsy. Epilepsia 58, 1972–1984.
- Elahian, B., Lado, N. E., Mankin, E., Vangala, S., Misra, A., Moxon, K., Fried, I., Sharan, A., Yeasin, M., Staba, R., Bragin, A., Avoli, M., Sperling,, M. R., Engel J., and Weiss, S.A. (2018). Low-voltage fast seizures in humans begin with increased interneuron firing. Ann. Neurol. 84, 588–600.
- Weiss, S. A., Sheybani, L., Seenarine, N., Fried, I., Wu, C., Sharan, A., Engel, J., Sperling, M. R., Nir, Y., and Staba, R. J. (2022). Delta oscillation coupled propagating fast ripples precede epileptiform discharges in patients with focal epilepsy. Neurobiol. Dis. 105928.
- Weiss, S. A., Pastore, T., Orosz, I., Rubinstein, D., Gornia, R., Waldman, Z., Fried, I., Wu ,C., Sharan, A., Slezak, D., Worrell, G., Engel, J., Sperling, M. R., and Staba, R. J. (2022). Graph theoretical measures of fast ripples support the epileptic network hypothesis. Brain Commun. 10.
- Weiss, S. A., Fried, I., Wu, C., Sharan, A., Rubinstein, D., Engel, J., Sperling, M. R., and Staba, R. J. (2023). Graph theoretical measures of fast ripple networks improve the accuracy of post-operative seizure outcome prediction. Sci. Rep.13, 367.
- Weiss, S. A. (2023). Chloride ion dysregulation in epileptogenic neuronal networks. Neurobiol. Dis. 177, 106000.
- Weiss, S. A., Eliashiv, D., Stern, J., Rubinstein, D., Fried, I., Wu, C., Sharan, A., Engel, J., Staba, R., and Sperling, M. R. (2023). Stimulation better targets fast‐ripple generating networks in super responders to the responsive neurostimulator system. Epilepsia, in press.